Chemistry Reference
In-Depth Information
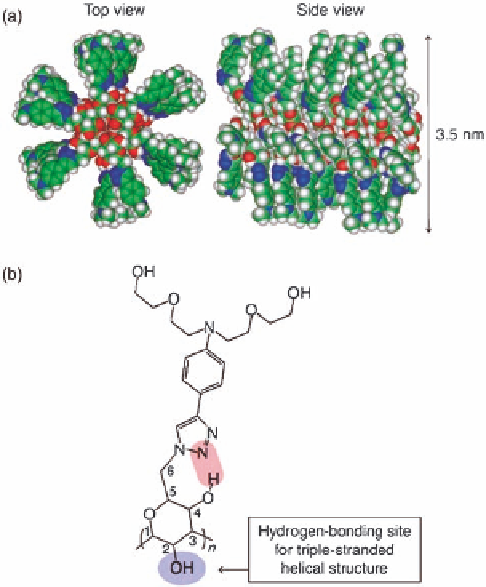
Figure 12.14 (a) Computer-generated Corey-Pauling-Koltun (CPK) model for the right-
handed, triple-stranded helical structure of CUR-DA. (b) Possible hydrogen-bonding mode
between glucose in the main chain and the 4-anilino-1,2,3-triazole moiety. The diethylene
glycol groups were substituted by methyl groups for clarity. Reprinted with permission from
[ref 114] Copyright 2007 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
conformational properties of SPG. It remained as a single-stranded random coil in
DMSO, for example, and formed triple-stranded helices with
20% water in DMSO
>
(Figure 12.14).
Normally, the triple-stranded helical structure of b-1,3-glucans is stable over a
wide range of temperature, pH, and ionic strength [99-101]. For
43
,theCDsignals
displayed small changes at higher temperatures and with up to 60mM of NaOH.
The remarkable stability is beneficial to the protection of the encapsulated contents
but problematic if one wants to release the contents. With the triazole groups in
the structure,
43
couldbindZn
2þ
and, interestingly, metal-binding was shown to dis-
sociate the triple helix. The authors suggested that the Zn
2þ
ions probably interfered
with the hydrogen-bonding interactions of the triazole moieties with the poly-
saccharide backbone and triggered the dissociation. The process was completely
reversible. When 1,4,8,11-tetraazacyclotetradecane (cyclam) was added to remove
Search WWH ::

Custom Search