Chemistry Reference
In-Depth Information
Most interestingly, both compounds displayed reversible changes in the UV-vis, fluo-
rescence, and CD spectra upon heating and cooling. When heated to 40
C, the UV
absorptions for a 0.1 wt% aqueous solution of
39
red-shifted by about 20 nm. A similar
red shift (about 10 nm) was observed for
38
. Heating also shifted the CD signals to the
red and decreased their intensity. These changes were consistent with lower excition cou-
pling within the aromatic stacks and a longer conjugation length. The cylindrical fibers
were shown by TEM to remain at the higher temperature but the cross-section became
5.5
0.4 nm or about 15% smaller in comparison to what was observed at the room tem-
perature. Meanwhile, the emission intensity increased significantly during heating, sug-
gesting a larger distance between the stacked aromatic groups in the helical fiber. These
results provided strong support for a more expanded helical structure upon heating and
were proposed to take place as the oligo(ethylene oxide) side chains became dehydrated
at elevated temperatures. This study is a remarkable example of how conformational
change in a foldamer can be exploited to produce novel environmentally responsive opti-
cal materials.
Schizophyllan (SPG) is a b-1,3-glucan that forms a triple helix in water that dissociates
into a single chain (s-SPG) in DMSO [99-101]. These polysaccharides were found by
Shinkai and coworkers to be excellent receptors for macromolecules [102]. SPG can form
macromolecular complexes with certain polynucleotides such as poly(C), poly(A), poly
(dA), or poly(dT) in water [103-105]. The binding involves hydrogen-bonding and
hydrophobic interactions among two s-SPG chains and one polynucleotide. Because the
complex spontaneously dissociates upon protonation of the nucleotide bases at pH
6,
the polymer is very promising for the endosomal delivery of polynucleotides [106].
SPG could also be modified at the glucose side chain to facilitate its membrane
permeability. The resulting materials were shown to deliver antisense DNA to cells effi-
ciently [107].
<
Other macromolecules that may be encapsulated within SPG helices include single-
walled carbon nanotubes [108] and conductive polymers [109]. SPG was shown to bind a
water-soluble polythiophene and force it to adopt a more planar conformation. During
binding, the conjugated polymer became chiral as it adopted a right-handed helical struc-
ture. The complex may be viewed as an insulated molecular wire wrapped by two poly-
saccharide chains [110].
The glucose side chain is important to the solubility of SPG. Once the side chain
is removed, the b-1,3-glucan (i.e., curdlan; CUR,
41
), has low solubility in most
solvents. By converting the primary alcohol into an azide, Shinkai and colleagues
were able to use the alkyne-azide click reaction [111-113] to install metal-binding
side chains onto the polysaccharide [114]. The polymer (
43
) retained many of the
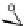




































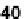



Search WWH ::

Custom Search