Chemistry Reference
In-Depth Information
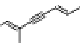
the silver complex
37
self-assembled into a 2-D hexagonal phase with a lattice con-
stant
a
29.6A
, but the helix seemed to be somewhat disordered. When the nitrate
wasreplacedbyBF
4
, a similar hexagonal phase was obtained with
a
¼
31.3 A
and a
second periodicity of 9.6 A
existed in the direction of the stacked hexagonal col-
umns. Calculations indicated that the compound formed helical structures with a
pitch of five repeat units. The helical pores were filled with the BF
4
counterions.
When the anion was triflate, X-ray diffraction and calculations were most consistent
with hexagonal stacks of cyclic dimers linked by two silver ions (Figure 12.13). The
even larger counterion, heptafluorobutyrate, prompted the transition to a lamellar
structure with a layer thickness of 32.2A
. It was proposed that the bulky anion could
no longer be contained within the pores formed by the
cis
-oriented aromatic back-
bones and forced the unfolding of the metallofoldamer chain into a zigzag structure.
Replacing the Ag
þ
ions in
37
with Pd
2þ
or Cu
2þ
and a slight elongation of the side
chain brought significant changes to the metallofoldamer in the solid state. The
Pd(II)-based coordination polymer formed lamellar structures with the pyridyl
ligands trans to each other in a square planar metal complex. The polymer based on
Cu
2þ
formed double helices through metal-chloride dimeric interactions [96].
¼
BF
4
) formed gels spontaneously above 2.5 wt% in water [97].
The addition of tetra-
n
-butylammonium fluoride (Bu
4
N
þ
F
) triggered depolymerization
of the coordination polymers due to the strong electrostatic interactions between Ag
þ
and
F
, turning the gel into a soluble mixture. The addition of Bu
4
N
þ
BF
4
gelled the solution
again. TEM analysis of the gels showed right-handed helical bundles of fibers with diam-
eters of 6-30 nm. These fibers entangled to form a network. The emission of the gel was
red-shifted and significantly weaker in comparison to that of the free ligand, supporting a
helical structure similar to that formed in the solid state. The gel-sol transition could also
be triggered by the addition of a larger ion, C
2
F
5
CO
2
. CD, fluorescence, and
19
FNMR
spectroscopy indicated that, instead of depolymerizaiton, the sol formation was caused by
the unfolding of the coordination chains into a zigzag conformation.
The Lee laboratory also reported two phenanthrene-based metallofoldamers
38
-
39
.
[98]. The fluorescence of the phenanthrene and the chiral side chain allowed them to char-
acterize the coordination polymers by multiple techniques. In comparison to the ligands,
the coordination polymers had the emission peak red-shifted by about 15 nm and signifi-
cantly quenched. The CD spectra showed strong signals for the aromatic chromophores,
Interestingly,
37
(A
¼
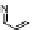





























Search WWH ::

Custom Search