Chemistry Reference
In-Depth Information
The transition was confirmed by X-ray crystallography, NMR spectroscopy, and electro-
spray mass spectrometry. A large change in the dimension of the molecular structure accom-
panied the metal-binding. As the helix unwound, the end-to-end distance of the oligomer
could increase by as much as 500% (Figure 12.8). The dimensional change was completely
reversible. Once the metal ions were removed by a stronger metal-binding azacryptand, the
linear structure reversed to the helix. Addition of triflic acid protonated the azacryptand and
released the metal ions to the py-pym oligomers, converting them to linear stands.
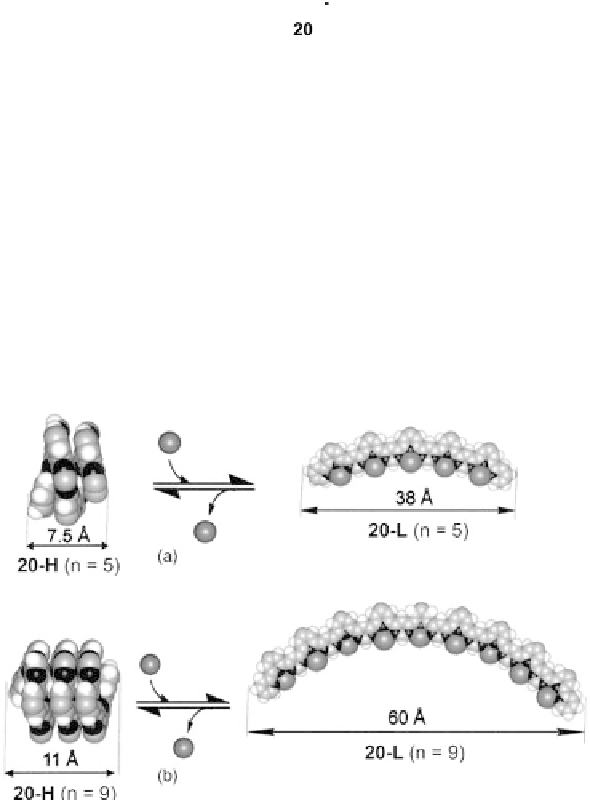
An impressive feature of biofoldamers is the enormous diversity in the structures
and functions created out of a relatively small set of building blocks. Such diversity
wasdemonstratedbyLehnandcoworkersin
20
. As the number of repeat units
decreased, the curvature of the unfolded conformer became smaller (Figure 12.9).
When
n
was reduced to 4, the curvature was small enough so that the lead ions on
one strand of the linear strand could complex with other strands to form molecular
grids (Figure 12.10). Depending on the amount of lead cations added, the compound
was shown to form either a [4
4] molecular grid or a [4 # 4] double-cross-
type architecture [83]. These are very different structures from the metal-free helices.
The transition could be viewed as a two-dimensional motion triggered by the ionic
signal.
Figure 12.9 Molecular models of the helical and extended rack-type complexes of 20 (
n
¼5
and 9). Reprinted with permission from [ref 78] Copyright 2002, National Academy of Sci-
ences, USA.
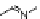



















Search WWH ::

Custom Search