Chemistry Reference
In-Depth Information
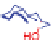
Thepresenceoftwothioethergroupsin
12
allowed it to bind mercury ions. In 2 : 1
hexane/EA with 5% methanol, Hg
2þ
quenched the dansyl emission efficiently and even
20 nM of Hg
2þ
could be easily detected by the fluorescence spectroscopy. The binding
was determined by the Job's plot to be 1 : 1. Unlike conventional sensors, however, the
mercury binding took place in the folded conformation of
12
, as the methionines were too
far apart in the unfolded conformer. Binding was extremely strong when the folded con-
former was favored by the solvents and very weak when the unfolded form was favored.
Experimentally, the binding constant between
12
and Hg
2þ
could be tuned over five
orders of magnitude by simple solvent changes [57].
The conformation of the sensor was the dominant factor controlling the binding affin-
ity. Interestingly, despite its relatively flexible nature, this foldamer demonstrated good
selectivity toward Hg
2þ
in comparison to other divalent cations such as Mg
2þ
,Zn
2þ
,
Cu
2þ
,Co
2þ
,Ni
2þ
, and even Pb
2þ
. Quite likely, in addition to the different binding affin-
ity, the quenching effect of the metal ion was also important to the selectivity. Mercury is
known, for example, to quench dansyl more strongly than most other metal ions [58].
Because of the large hydrophobic surface of an oligocholate, even multiple
hydroxyl and amide groups are insufficient to give any water solubility to the fol-
damer. The Zhao group found that oligocholates could be solubilized by common
surfactant micelles in water and the micelles greatly impacted the conformations of
the foldamers [56,59,60]. Foldamer
12
couldbeincludedwithinthemicellesofsur-
factants of many types, including the cationic cetyltrimethylammonium bromide
(CTAB), anionic sodium dodecylsulfate (SDS), and nonionic Triton X-100 [61]. The
environmentally sensitive emission of the dansyl group made it easy to study the
conformation of the oligocholate in micelles. From the emission properties of the
micelle-solubilized
12
, it was clear that nonionic Triton X-100 formed micelles with
the most hydrophobic interior. The charge of the surfactant impacted the binding
strongly, following the order: cationic micelle
anionic
micelle. The different bindings were attributed to the electrostatic interactions
between the positively charge Hg
2þ
and the micelle [61].
The Zhao group later reported dicarboxylated oligocholate
13
[62]. The foldamer
was functionalized with two pyrenyl groups at the chain ends. Due to its long fluo-
rescence lifetime, pyrene could form excimers quite readily [63]. The folding of
13
,
therefore, could be detected by the excimer formation at 470 nm in the fluorescence
spectrum. The two carboxylic acid groups of
13
helped the folding significantly.
Whereas the parent hexamer could not fold in the binary methanol/EA mixture at
all,
13
couldfoldwithupto15%methanol.When
13
was titrated with zinc acetate,
the excimer/monomer ratio underwent characteristic changes. In 5 and 10% methanol,
the compound was already folded and the addition of Zn
2þ
caused very little change to
the excimer/monomer ratio. Although the addition of Zn
2þ
enhanced the excimer
formation above 15% methanol, the enhancement was the largest with 15% methanol.
nonionic micelle
<<
<





















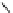




























































Search WWH ::

Custom Search