Chemistry Reference
In-Depth Information
12.3 Metallofoldamers as Sensors for Metal Ions
Molecular recognition is the corner stone of supramolecular sensing. When a specific
binding event is expressed in a readable signal, the host has the potential to become a
sensor. Of course, to be truly qualified as a sensor, the supramolecular host needs to bind
the target analyte with high specificity and affinity, preferentially in the presence of com-
peting species.
Foldamer and nonfoldamer sensors are similar in that both possess a molecular
recognition site and a signal-generating site. The latter is commonly a chromophore or
fluorophore that undergoes spectroscopic changes upon binding with the analyte. What
distinguishes a foldamer and a nonfoldamer sensor is the former's conformational change
during the binding. As mentioned in the introduction of the chapter, changes in the physi-
cal and/or chemical properties often accompany the conformational change of a molecule,
a feature immensely useful to the design of sensors.
An early example of a foldamer-based sensor was reported by Ajayaghosh and col-
leagues [43]. Molecule
11
has two squaraine dyes connected by a flexible oxyethylene
tether. The unfolded conformer had its maximum absorption at 630 nm in acetonitrile.
The addition of Na
þ
and K
þ
ions affected neither the absorption nor the emission of the
compound but the addition of Ca
2þ
turned the color of the solution from light blue to
intense purple-blue. In the UV-vis spectrum, the peak at 630 nm decreased while a new
peak at 552 nm appeared. The fluorescence of the compound (l
max
¼
652 nm) was signifi-
cantly quenched. Importantly, similar alkaline earth metals such as Mg
2þ
,Sr
2þ
, and Ba
2þ
brought minimal changes to the absorption and emission spectra of the compound. The
changes were proposed to occur as a result of the face-to-face stacked conformation.
The arrangement of the dyes resembled the “H” aggregates of squaraine dyes, which
tend to display blue-shifted absorptions compared to the monomers. The folding was
found to be reversed by the addition of EDTA that removed the metal ion from the folded
structure [44].
Molecule
11
is unable to fold on its own. Its metal-assisted folding was mainly
exploited to generate the signal. If the sensor itself is able to fold, will its conformational
property affect its ability to bind the metal? Zhao and coworkers addressed this question
in a series of works, with their oligocholate foldamers (Figure 12.5).
The oligocholates were prepared from cholic acid [45], a widely used building block
for supramolecular receptors [46-53]. Their folding is driven by an unusual type of solvo-
phobic interactions [54-56]. When dissolved in a largely nonpolar solvent mixture, the
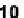



































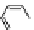







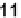




























































Search WWH ::

Custom Search