Chemistry Reference
In-Depth Information
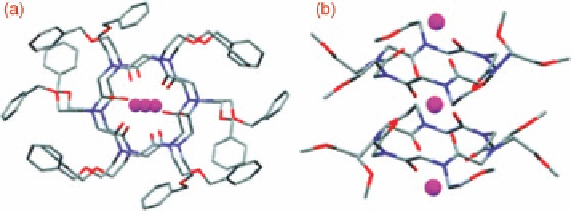
Figure 11.24 X-Ray crystal structure of 13
2
[Sr(Picr)
2
]
3
complex. (a) Top view. (b) Side view.
Hydrogen atoms and picrates have been omitted for clarity. [56] Reproduced by permission of
The Royal Society of Chemistry.
equilibrium NMR studies indicated that the electrostatic (ion-dipole) interactions stabi-
lize this conformation in solution. The authors reported one successful attempt which pro-
duced needle-like crystals, suitable for X-ray structure analysis, of the cyclic hexamer as a
2 : 3 complex with strontium picrate (Figure 11.24). The analysis showed a unique peptoid
bond configuration with the carbonyl groups alternately pointing toward the strontium
cations and forcing the N-linked side chains to assume an alternate pseudo-equatorial
arrangement. Although the authors do not discuss conformational control in cyclic pep-
toids upon metal coordination, this work was found to be interesting in the context of this
chapter because, to date, it reports the first and only example of a metallopeptoid structure
to be solved. Moreover, in a follow-up study, these laboratories reported cation transport
carried by cyclic peptoids across a phospholipid membrane [59]. Two additional cyclic
peptoids - an octamer and a decamer - were synthesized and characterized and their size-
dependent selectivity for first group alkali metal cation transport was demonstrated.
11.5 Concluding Remarks
The selected examples of metallofoldamers discussed here illustrate the significant prog-
ress made in the design of foldamers that adopt well defined secondary structures upon
metal-ligand coordination interactions in combination with a variety of other interactions,
such as hydrogen bonding, aromatic p-stacking and solvophobic effects. Moreover, met-
allofoldamers have shown an impressive ability to form single-handed helical structures
and other chiral architectures. The synergistic interaction between helices and metal com-
plexes in metallopeptoids demonstrates the transfer of chiral information from a folded
scaffold to an embedded metal center and, hence, has a potential for applications in asym-
metric catalysis.
References
1. Hill, D.J., Mio, M.J., Prince, R.B.
et al.
(2001)
Chem. Rev.
,
101
, 3893-4011.
2. Gellman, S.H. (1998)
Acc. Chem. Res.
,
31
, 173-180.
Search WWH ::

Custom Search