Chemistry Reference
In-Depth Information
found in biological systems. This work is a prominent example of a tertiary structure for-
mation governed by the docking of pre-organized helices.
Maayan
et al.
designed and synthesized helical peptoids bearing one or two multiden-
tate ligands. Upon metal coordination, an enhancement of the secondary structure was
demonstrated, either by stabilization of an existing helix or by the formation of a helical
duplex. Moreover, the helical secondary structure environment induces chirality about the
metal center and enforces the creation of a chiral metal complex from ligands which are
not inherently chiral [52].
The introduction of multidentate metal-binding ligands as pendant groups in non-heli-
cal peptoid sequences was first demonstrated and presented in an earlier study by the
same group [53]. For example, 8-hydroxy-2-quinolinemethylamine was prepared from
the commercially available 8-hydroxy-2-quinolinecarbonitrile by a one-step hydrogena-
tion procedure and incorporated within different peptoids without any need for protection
of the hydroxyl group. In order to explore the influence of metal binding on the conforma-
tion of chiral helical peptoids, hydroxyquinoline ligands were incorporated to their scaf-
folds. These peptoid ligands were expected to bind divalent metal ions, producing tetra-
coordinated metal species [54]. Specifically,
H
1
5
and
H
2
6
(Figure 11.21) were synthe-
sized as model systems for a comparison of inter- and intramolecular metal complex for-
mation, respectively.
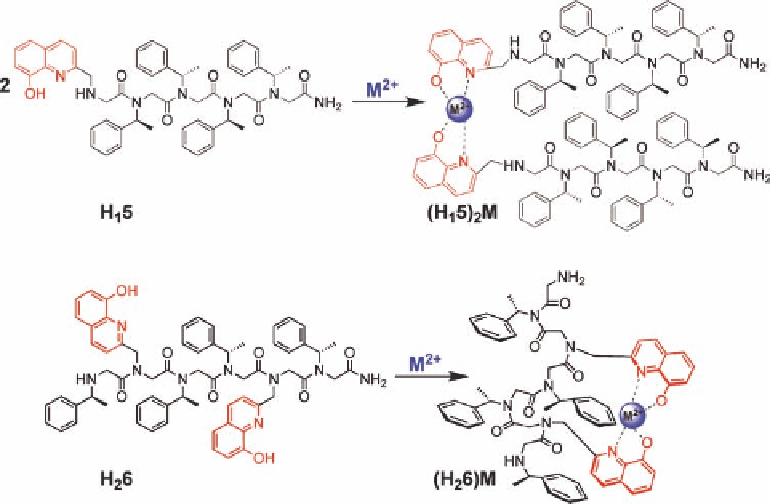
The pentamer
H
1
5
, with one hydroxyquinoline site at the N-terminus, was expected to
form a peptoid duplex upon metal binding (2 : 1 peptoid:metal). The hexamer
H
2
6
con-
tains two hydroxyquinoline ligands, endowing this oligomer with the capacity to form an
Figure 11.21 Schematic representations of the peptoids H
1
5 and H
2
6 and their metal com-
plexes [52].
Search WWH ::

Custom Search