Chemistry Reference
In-Depth Information
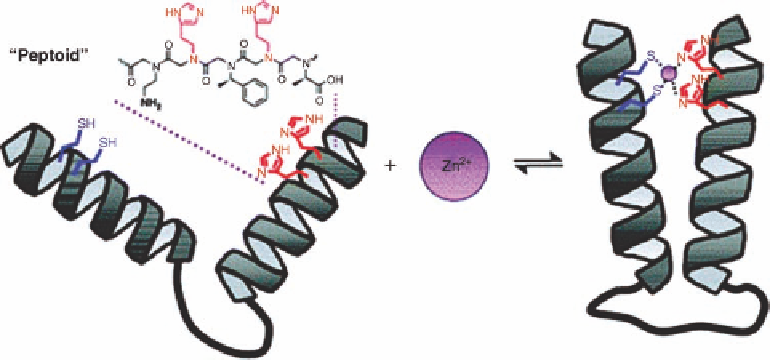
Figure 11.20 Schematic representation of peptoid two-helix bundle forming upon Zn
2þ
binding. Reprinted with permission from Ref. [49]. Copyright 2008 American Chemical
Society.
(1-carboxylethyl)glycine (Nsce) - impose steric hindrance on the backbone, generating a
polyproline type I-like helix with three residues per turn. The chosen motif for the loop
region was Gly-Pro-Gly-Gly, which has a propensity to form type II b-turns in proteins
[50]. Borrowing from well understood zinc-binding motif Cys
2
His
2
[45a,b], thiol and
imidazole moieties were positioned within the peptoid such that both helices must align
in close proximity to form a binding site. In order to measure the change of the distance
between the two helical segments and to probe the binding of zinc, fluorescence reso-
nance energy transfer (FRET) reporter groups were used [51]. Thus, a fluorescence donor
(anthranilamide) was incorporated at one end of the peptoid and a quencher (nitrophenol)
at the other end. FRET efficiencies were used to determine the folding of the peptoids and
the affinity and selectivity of the zinc-binding peptoids. The first observation was that zinc
binding increases the FRET efficiency in acetonitrile, indicating that zinc stabilizes the
two-helix bundle by holding the two helical segments together. Moreover, in mixtures of
two different peptoids, there was no change in the intermolecular FRET efficiencies upon
the addition of zinc, proving that the two helix bundles are stabilized without inducing
self-association. CD spectroscopy revealed no significant changes in the far-UV region
upon zinc binding, indicating that the secondary structure of the individual peptoid
helixes is unchanged. In addition, the position and number of zinc-binding residues, as
well as the sequence and size of the loop that connects the two helices were systemati-
cally varied, followed by FRET efficiency measurements. The results suggest, for exam-
ple, that a higher zinc-binding affinity was due to a longer loop region in the peptoid,
probably because longer flexible linkers accommodate optimal zinc-coordination geome-
try. In addition, among various divalent metal ions (e.g., Ca
2þ
,Mg
2þ
,Cu
2þ
)themost
significant change in the FRET efficiency was achieved by Zn
2þ
, with a binding affinity
an order of magnitude higher than the other metal ions, a kind of selectivity that is also
Search WWH ::

Custom Search