Chemistry Reference
In-Depth Information
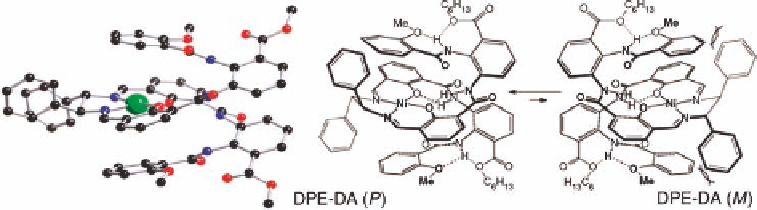
Figure 11.15 X-Ray crystal structure of DPE-DA (left) and the rational for its preference for
the (
P
)-helical conformer (right). [33] Reproduced by permission of The Royal Society of
Chemistry.
derived from those oligomers were compared both in solution and in the solid state. NMR
studies in solution indicated that the metallofoldamers have substantially higher field chemi-
cal shifts relative to the salicylaldehyde starting material. These upfield chemical shifts are
consistent with a helical structure in solution. CD spectra of the three metallofoldamers were
also obtained and the intensity of their peaks was evaluated and compared to the CD peak
intensity of the metallofoldamer
5TCDA
, which has positive dichroisms at 422 and 445 nm.
It was found that the CD spectra of
CP-DA
and
DEA-DA
were very weak and there was
almost no bias towards one helical conformation. In contrast,
DPE-DA
affords a CD spec-
trum which resembles that of
5TCDA
, with negative dichroisms at 428 and 456 nm and
only 25% less intensity. This CD spectrum suggested that
DPE-DA
is composed mainly of
a(
P
)-helical conformation, an assumption that was further confirmed by the crystal structure
of
DPE-DA
that was determined and identified as the (
P
)-helix [33]. Subsequently, the salen
metallofoldamers were studied by variable temperature NMR in order to assess the confor-
mational ratios between the (
P
)- and (
M
)-helices. According to the obtained data, it was
concluded that
DPE-DA
shows a large preference (6 : 1) for the (
P
)-helix, while
CP-DA
shows only a slight preference (2 : 1) for the (
M
)-helix and for
DEA-DA
the helical diastere-
omers are present in similar amounts. In order to rationalize the relatively large bias for the
(
P
)-helical conformation in
DPE-DA
the authors propose that the (
M
)-helical conformation
of
DPE-DA
suffers a steric interaction between the terminal anisoyl groups and the phenyl
substituents from the diamine. These steric interactions are relieved in the (
P
)-helical con-
formation (Figure 11.15). Overall these studies illustrate how different internal chiral dia-
mines affect the equilibrium between helical diastereomers in salen-based foldamers.
11.4 Folded Oligomers in Which Metal Coordination Enhances Secondary
Structure and Leads to Higher-Order Architectures
One of the long-term goals in the development of functional folded materials is the cre-
ation of stable structures with protein-like properties. Despite recent advances in the sta-
bilization of secondary structures upon metal coordination, the design of a sequence that
can fold into a well defined tertiary structure in solution is still challenging. The focus of
ongoing studies aiming at the generation of folded architectures by metal coordination
is threefold: (i) enhanced stabilization of an existing secondary structure (e.g., helix),
Search WWH ::

Custom Search