Chemistry Reference
In-Depth Information
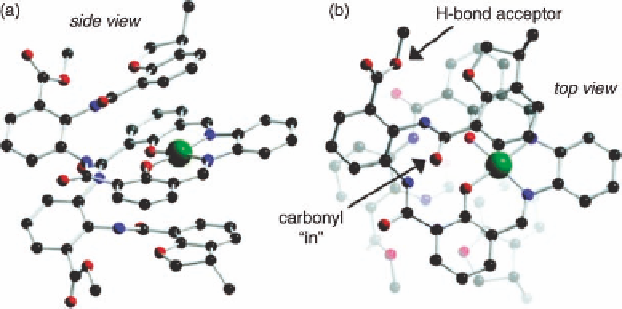
Figure 11.12 Molecular structures of a chiral foldamer from crystallographic coordinates.
Reprinted with permission from Ref. [27]. Copyright 2006 American Chemical Society.
into the “carbonyl-in” (
M
)-helix conformation (Figure 11.12). Evidence that the solution
structure of
5
is helical was provided by CD and
1
H-NMR measurements. Based on the
results, the authors point out that either
5(
M
)
is the major conformation, or that the
undetermined conformer also has
M
-helicity. Overall, this work demonstrates that control
over the absolute sense of helicity can be effectively achieved by a combination of hydro-
gen bonding and steric interference arising from peripheral stereo-centers in a metal coor-
dination derived foldamer.
The role of peripheral stereo-centers - chiral end groups - as directors of absolute hel-
icity in salen- and salophen-based metallofoldamers was further investigated by the Fox
group [30-32]. Initial studies showed that the chiral end group in metallofoldamers
4
and
5
are, in fact, the components responsible for the foldamer's absolute helicity. Even when
trans
-cyclohexane-1,2-diamine (TCDA), which is a common element of central chirality
in salen catalysts, was embedded within oligomer
6
, the absolute helicity of its nickel
complex was directed by the chirality of the end groups rather than the chirality of the
central diamine group. This was proven by using an oligomer that contains both the
TCDA group with an
S
,
S
chirality [bias for a (
P
)-helix] and end groups that are bias for a
(
M
)-helix (oligomer
6TCDA
, Figure 11.13a) and demonstrating that the nickel complex
5TCDA
is a (
M
)-helix (Figure 11.13b) [30,31]. Moreover, the Fox group advocates that
the ability of end groups to enforce absolute helicity does not necessarily require the
incorporation of large and bulky groups; and they demonstrated that small chiral end
groups could also control the absolute sense of helicity in salen and salophen metallofol-
damers [32]. In their study, chiral 1-methylindan compounds were used as end groups and
the new oligomers produced chiral metallofoldamers upon coordination to nickel ions.
Spectroscopic studies in solution and NMR data, as well as an X-ray diffraction analysis
performed on solid crystals of the metallofoldamers, revealed that these complexes adopt
a helical structure with an absolute sense of helicity, which is dominated by the stereo-
centers at the periphery [32]. Therefore, it can be concluded that the presence of chiral
end groups, large or small, in metallofoldamers could play a crucial role in controlling
their absolute helicity.
Search WWH ::

Custom Search