Chemistry Reference
In-Depth Information
O
O
N
N
N
H
H
O
O
O
O
a b c
O
O
HN
NH
R
R
H
2
(
2
), R = a - c
Figure 11.9
Structure of chiral 2,6-bis[(2-carbamoylphenyl) carbamoyl]pyridine ligands,
H
2
(2a-c) [21].
Deprotonation of the pyridyl amides facilitated the ability of the H
2
(
2
) ligands to bind
metal ions. The solid-state structures of these metal complexes was examined by an X-ray
diffraction study conducted on H
2
(
2b
). This study revealed that a nickel complex is crys-
tallized with two independent molecules, Ni(
2b
0
)andNi(
2b
00
), in the asymmetric unit
cell. Preliminary NMR studies suggest that the asymmetric arrangement of the appended
arms found in the solid-state structure of Ni(
2b
) can be maintained in solution.
Unfortunately, the Borovik group did not report chiral metal-helical compounds that
maintain their structure in solution. Moreover, the molecules depicted in Figure 11.9 are
capable of forming helical structures in solution as a result of their hydrogen-bonding net-
work; hence, folding does not occur specifically upon metal binding. Metal coordination,
as demonstrated in the above examples, can nucleate a helical structure but does not have
a significant role in generating a new helix in solution.
The challenging task of nucleating a new helical structure was implemented by the
group of Fox [6] using abiotic molecules based on salophen and salen ligands. Although
these molecules do not adopt helical structures in the absence of metal ions, they were
shown to fold into single-stranded helices in the presence of Ni
2þ
or Cu
2þ
(Figure 11.10).
Figure 11.10 Abiotic oligomers for the examination of hydrogen bonding and coordination
interaction. Reprinted with permission from Ref. [6]. Copyright 2005 American Chemical
Society.








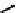


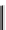











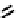







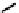



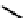
















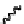


















Search WWH ::

Custom Search