Chemistry Reference
In-Depth Information
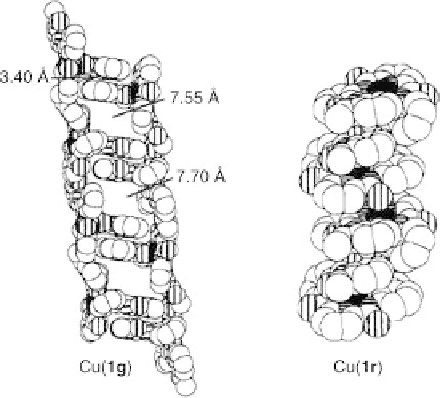
Figure 11.7 Space-filling representation of Cu(1g) (left) and Cu(1r) (right). The solvent mole-
cules and non-amide hydrogens are removed for clarity. Reprinted with permission from
Ref. [19]. Copyright 1996 American Chemical Society.
In contrast, in the case of Cu(
1r
), the lack of one Cu-O bond leads to a
realignment of the appended arrays and produces a more compact helix, which does
not have a porous morphology (Figure 11.7, right); the helical molecules of Cu(
1r
)
associate to form longer, extended helices in the solid state (Figure 11.3). Each
extended helix consists of alternating right- and left-handed helical monomers that
assemble through intermolecular p-stacking interactions. Although these results are
only valid in the solid state [the structural properties of Cu(
1g
)andCu(
1r
)areiden-
tical in solution], it is possible to conclude that metal coordination to H
2
(
1
) enables
the nucleation of helical structures and the generation of new helices which can be
altered upon variations in the metal ion precursor.
Studies on the complexes Cu
1
[including both Cu(
1g
) and Cu(
1r
)], as described above,
suggested that the presence or lack of Cu-O amide bonds is a highly important factor for
determining the final structure of the produced helices. To further investigate possibilities
in which the helical structure could be modified, Borovik's group was interested in find-
ing ways to control the formation of these Cu-O bonds. Thus, Cu
1
was treated with two
different ligands, pyridine and lutidine, which are basic enough to substitute the oxygen
amide groups [20]. Changes in the spectroscopic properties of Cu
1
upon binding to either
pyridine, forming Cu
1
(py), or lutidine, forming Cu
1
(lut), implied that the coordination
environment about the Cu
2þ
centers has been altered. An X-ray diffraction study on
Cu
1
(py) confirms that the binding of a single pyridine to copper causes a significant struc-
tural rearrangement. The two oxygen amide donors that were coordinated originally to the
copper in Cu
1
are rotated away from the copper in Cu
1
(py) and no longer interact with
the metal ion (Figure 11.8, left). Consequently, a chiral cleft about the exogenous pyridine
is formed by the appended groups in H
2
(
1
)
2
. An interesting characteristic of the Cu
1
(py)
crystal structure is that all the complexes within the lattice have the same helical chirality.
Search WWH ::

Custom Search