Chemistry Reference
In-Depth Information
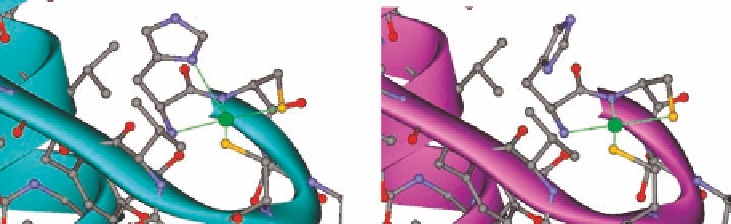
Figure 1.17 The N-terminal Ni-binding active site of Ni-SOD in the oxidized Ni
3þ
form (left,
PDB ID 1Q0D) and the reduced Ni
2þ
form (right, PDB ID 1Q0K).
additional axial ligand from His1 residue in the oxidized Ni
3þ
state (Figure 1.17) [224].
Here, the axial His seems to serve as a redox and/or conformational switch of the N-termi-
nal active-site Ni coordination of this enzyme, detaching from the metal to stabilize the
Ni
2þ
state via a lowering of the d
z
2 energy level and binding to the metal to donate further
electron density to Ni
3þ
. A large conformational change during the redox cycle may not
take place so that electron transfer is not slowed down by the conformational movement.
This catalytic cycle is thus a good example for demonstrating the significance of the con-
formation and coordination of active-site metal in retaining the proper function of
“natural metallofoldamers.”
The correlation between conformational change and redox activity of Ni-SOD has been
modeled with the simple peptide H
2
N-Gly-Cys-OMe, which folds to bind to Ni
2þ
via the
N-terminus, deprotonated amide, and Cys side chain S, as well as a couple dipeptide
mimics to form complexes with a coordination sphere of N
2
S [225]. Various external thi-
olates are introduced to the complexes to complex the square-planar coordination sphere
of N
2
S
2
as in reduced Ni-SOD. The coordination sphere and reactivity of Ni-SOD is also
mimicked with the Ni
2þ
complex of the tripeptide Asn-Cys-Cys with a coordination
sphere of N
2
S
2
(including a deprotonated amide), as in Ni-SOD, consistent with a dia-
magnetic Ni
2þ
in square-planar geometry [226]. This complex undergoes chiral structural
transformation that is associated with its SOD activity.
1.3.3.2 Metal-Triggered Conformational Change in Peptides
Helical coiled-coil structures can be designed on the basis of the tendency of amino acids
to form helix structures [227] and inter-stranded interactions. However, a peptide
designed to form a double-stranded parallel coiled-coil structure ended up showing triple-
stranded “up up down” a-helices [228], reflecting the great conformational flexibility of
peptides and their high degree of freedom in assembling into higher-order structures. Syn-
thetic peptides can afford a helical conformation upon metal binding to metal-binding
sequences such as His-x-x-x-His and Cys-x-x-x-His [229], consistent with an a-helical
i
-(
i
4) conformation, or to moieties with unnatural metal-binding ligands [230]. Short
peptides can also be triggered to form a-helices with metal ions by the use of such a
method [231], which can be as short as only one helical turn [232]. Despite their similar
ligand-binding capabilities, Ni
2þ
and Cu
2þ
ionswerefoundinonecasetoinduce
þ
Search WWH ::

Custom Search