Chemistry Reference
In-Depth Information
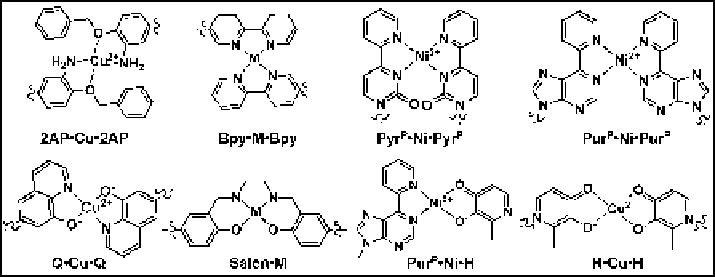
Figure 10.7 Alternative base pairs based on metal complexes that contain a pair of bidentate
ligands.
entry 2). For incorporation in ss PNA,
Bpy
(or 5-methyl-2,2
0
-bipyridine) has been con-
nected at its 5
0
-position to the secondary NH group in the Aeg backbone through a CH
2
-
CO linker (Table 10.1, entry 3) [16a,c,50]. We note that the properties of dsPNAs contain-
ing 2,2
0
-bipyridine or 5-methyl-2,2
0
-bipyridine were similar [16c]. Two other ligands that
have the same cis-diimine metal binding site as 2,2
0
-bipyridine have been used also to
form metal complexes that function as alternative base pairs in DNA duplexes [13a,b]. 4-
(2
0
-pyridyl)-pyrimidinone (
Pyr
P
; Table 10.1, entry 4) and 6-(2
0
-pyridyl)-purine (
Pur
P
;
Table 10.1, entry 5) are formally obtained by substitution with pyridine of the 4-amino
group of cytosine and of the 6-amino group of adenine, respectively (Figure 10.7).
Substitution of a nucleobase pair in the middle of a DNA or PNA duplex with a pair of
Bpy
,
Pyr
P
or
Pur
P
ligands reduced the thermal stability of the duplexes below that of the
corresponding, non-modified duplex, which can be related to the fact that these ligands do
not form H-bonds within the pair [13a,b,16a,c]. Nevertheless, there are cases of both
DNA and PNA duplexes in which
Bpy
did not affect the melting temperature [27b,c,49].
The decrease in the melting temperature of 12-bp DNA duplexes containing
Pur
P
ligands
was smaller than that for duplexes with the same sequence with
Pyr
P
ligands, which
could be due to better p-stacking of the larger
Pur
P
ligands [13a,b].
The metal-based alternative base pairs influence the properties of the nucleic acid
duplexes and the duplex context influences the properties of the metal complexes. It
is difficult to identify simple cause-effect relationships between these factors and the
properties of nucleic acid duplexes because of the limited number and diversity of
systems studied this far. Nevertheless we identify below several correlations between
the properties of the metal complex and of the duplexes. These correlations can be
considered in the design of new metal-containing duplexes for specific synthetic or
functional goals.
1. The way in which a given ligand is attached to the nucleic acid backbone, that is, the
position in the ligand where the attachment is made, and the chemical nature of the
linker between the ligand and the backbone of the nucleic acid can influence the effect
of the ligand and metal complex on the duplex.
Search WWH ::

Custom Search