Chemistry Reference
In-Depth Information
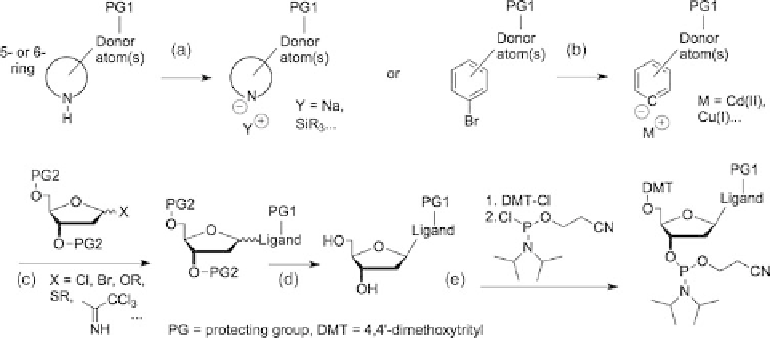
Figure 9.6 General overview of the synthesis of ligand-modified nucleosides. (a) activation
for
N
-glycosylation; (b) activation for
C
-glycosilation; (c) reaction with glycosyl donors;
(d) separation of anomers and deprotection; (e) attachment of protecting and activating groups
for automated DNA synthesis.
In a convergent synthetic approach, an
N
-nucleophilic (Figure 9.6a) or
C
-nucleophilic
(Figure 9.6b) ligand-modified nucleobase carrying suitable protecting groups must be pre-
pared and subsequently brought into reaction with a glycosyl donor (a 3
0
O
-/5
0
O
-protected,
1
0
-activated 2
0
-deoxyribosyl electrophile) such as Hofmann's a-1
0
-deoxyribosylchloride
to yield the nucleoside, usually as a mixture of a-andb-anomers (Figure 9.6c). After-
wards, the desired b-anomer has to be separated from the undesired a-anomer (usually
by chromatography or recrystallization) and the protecting groups are removed from the
3
0
O
- and 5
0
O
-positions (Figure 9.6d). Care has to be taken in the unambiguous characteri-
zation of the two anomeric isomers (e.g., by NOESY-NMR spectroscopy or single-crystal
structure determination) to make sure that indeed the desired anomer ends up in the final
DNA product.
Finally, the 5
0
O
-position is equipped with the 4,4
0
-dimethoxytrityl (DMT) protecting
group and the 3
0
O
-position is phosphorylated with 2-cyanoethyl-
N
,
N
-diisopropylchloro-
phosphoramidite to yield the phosphoramidite building block needed for the standard pro-
tocol of the automated solid-phase DNA synthesis (Figure 9.6e) [7]. Both latter reagents
have been specifically developed for automated DNA synthesis and we shall see later
(Section 9.3.4) the reason for this. Since the product contains an acid labile DMT group
and a phosphorus(III) center very prone to hydrolysis and oxidation to a phosphorus(V)
by dioxygen, special care has to be taken during synthesis and purification and the final
product should be checked by
1
H- and
31
P-NMR spectroscopies.
The commercially available phosphoramidite building blocks for the natural nucleo-
bases A, G and C (but not T) carry protecting groups on nucleophilic positions of the
bases that survive the conditions of DNA synthesis but are smoothly cleaved after DNA
synthesis to release the fully unprotected oligonucleotide. Similar considerations have to
be made for any protecting groups attached to the artificial nucleobase: whereas their
main purpose is to protect the nucleophilic (or electrophilic) sites on the ligand from
Search WWH ::

Custom Search