Chemistry Reference
In-Depth Information
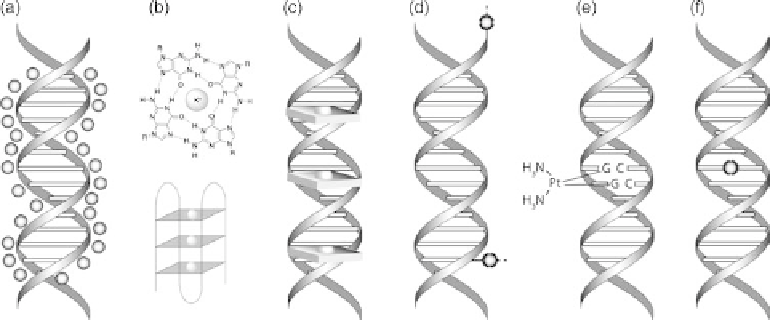
Figure 9.3 Types of DNA metal interactions. (a) Unspecific backbone/ groove binding for
charge compensation; (b) binding of K(I) in the centre of G-quadruplex structures; (c) groove
binding/ intercalation of metal complexes; (d) specific coordination of metals to artificial lig-
ands attached to the ends or sides of DNA strands; (e) inter- or intrastrand crosslinking of natu-
ral nucleobases by coordination compounds such as
cis
-platin; (f) metal base pairing in the
centre of the DNA double helix.
with the negatively charged phosphate groups along the backbone for reasons of charge
compensation and hence structural stabilization (although it must be noted that ammo-
nium ions or related biological compounds such as spermine serve a similar job) [2,21].
The G-quadruplex motif found in the telomeric G-rich repeats was found to be stabilized
by K(I) ions sitting in the central cavity between each of the G
4
squares (Figure 9.3b)
[22]. The intercalation (and/or groove binding) of metal complexes such as [Ru(bpy)
3
]
2þ
and related structures inside the base stack of double-stranded DNA has relevance for
the research on electron conductance through DNA, the use of DNA as chiral ligand in
enantioselective catalysis [23] and the development of DNA targeting therapeutics
(Figure 9.3c) [24]. Systems capable of binding metal ions selectively by ligands attached
covalently to the ends or sides of DNA strands have been investigated for new ways of
metal-mediated DNA nanoconstruction and their ability to enhance the conductivity of
DNA (Figure 9.3d) [25]. Metal complexes directly binding to the natural nucleobases by
coordination bonds are most prominently exemplified by the complex formed between the
cytostatic drug
cis
-platin and two neighboring guanine bases via the coordination of both
of their N7-nitrogen atoms (Figure 9.3e) [26]. Finally, metal ions can be coordinated right
inside the core of the DNA double helix either by replacing the protons in the hydrogen
bonds holding together the pairing bases in natural Watson-Crick base pairs or between
the donor atoms of artificial ligands built inside the DNA [20].
9.2 The Quest for Alternative Base Pairing Systems
Enhancing the intrinsically low electron conductivity of natural DNA by doping the
strands with metal ions is one motivation that led to the development of metal base pairs,
Search WWH ::

Custom Search