Chemistry Reference
In-Depth Information
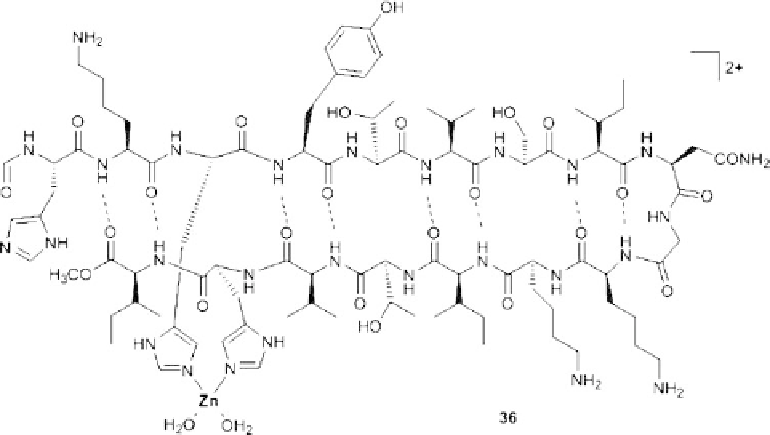
Figure 8.23 A zinc(II) stabilized b-hairpin.
The addition of copper(II) ions to
35
results in the formation of a 1 : 1 complex in
which the short peptidic moieties are orientated parallel to each other. This is supported
by an additional metal coordination of carbonyl units neighboring the coordination site,
resulting in the favored square planar coordination at the metal. The peptide units now
easily undergo hydrogen bonding and an anti-parallel b-sheet with five intramolecular
hydrogen bonds is formed. In principle, the bipyridine copper complex of
35
mimics a
turn leading to the preorganization of the two peptidic segments for non-covalent interac-
tion [60].
An alternative for the metal-assisted b-sheet formation of
35
can be found in the
b-hairpin structure
36
. Two histidine residues in the peptide are ideally located in the
strand in order to bind zinc(II) ions (Figure 8.23). This results in a huge macrocycle,
which by hydrogen bonding adopts a b-sheet structure. Here, the turn unit, composed of
the Asn-Gly dipeptide, is located far from the metal coordination sites [61].
In the two discussed examples shown for the stabilization of b-sheet structures differ-
ent approaches are used. The strands are forced close to each other either by the introduc-
tion of a turn mimic or by tethering the termini of the peptide by metal coordination. In
either case, the stabilization of the structures relies on cooperative effects between the
binding of the metal ions and the formation of hydrogen bonds.
8.5 Conclusion
Metal coordination is an important tool to assemble and stabilize well defined structures
and networks. It is utilized for the self-assembly of metallosupramolecular structures
which can reach high complexity. The introduction of amino acids or peptidic units allows
Search WWH ::

Custom Search