Chemistry Reference
In-Depth Information
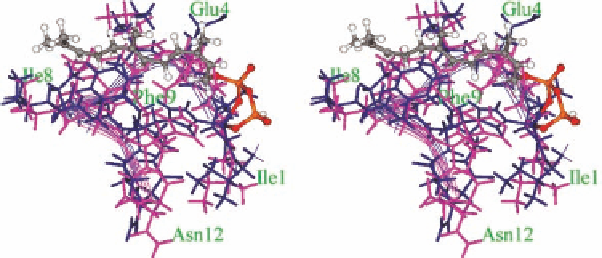
Figure 1.12 The structures of Bc-metal complex (pink) and a model of the ternary complex
(blue) upon the binding of farnasylpyrophosphate (ball and stick) to metallo-Bc. Conforma-
tional changes due to formation of the metal complex and ligand binding are clearly seen
including Phe9 and possible detachment of coordinated Glu4.
1.3.1.3 Antibiotic Salivary Peptide
The histatin (Htn) family is comprised of His-rich salivary peptides in higher primates
[137-140], showing antibiotic activities against
Streptococcus mutans
[141] and
S. mitis
[142],
Saccharomyces cerevisiae
[143],
Cryptococcus neoformans
[144], and
Porphyro-
monas gengivalis
, as well as the opportunistic pathogenic yeast
Candida albicans
[145].
Htn5 has the highest concentration among the Htns in human saliva. It is the first
24 amino acids of Htn3 (DSHAK RHHGY
10
KRKFH EKHHS
20
HRGY) and exhibits its
highest activity against
C. albicans
[138]. Htn5 does not form pores in the bacterial cell
membranes [146] but internalizes by binding to the heat shock protein Ssa1/2 on the cell
wall [147], followed by interaction with the K
þ
transporter TRK1 [148] which leads to
apoptosis [149]. It may be internalized into mitochondria and interfere with the electron
transfer processes to cause cell death [150].
Htn5 contains several potential metal-binding residues from His, Asp, Glu, and Tyr, has
been shown to bind divalent metal ions in the order of Cu
2þ
>
Ca
2þ
Fe
2þ
[151], and is suggested to bind three equivalents of Zn
2þ
or Cu
2þ
from a calorimetric
study. Cu
2þ
and Ni
2þ
bind Htn5 at the N-terminal DSH with a high affinity analogous to
DAH in bovine serum albumin which folds the N-terminus [152] to afford a square planar
coordination geometry on the basis of electronic, NMR, and EPR results [153], and poten-
tially also at HEXXH and two His-His sites, as found in many metallopeptides and metal-
loproteins. However, Co
2þ
seems to bind to Htn5 first with three His residues and with two
His residues in the second site [153]. Metal binding of Htn5 plays an important role in its
bioactivities by fusing negatively charged vesicles (Zn
2þ
binding [151a]) and exhibiting
oxidative nuclease activity (Cu
2þ
binding [154]). Similar to many Cu
2þ
complexes, the
complex Cu
2
2þ
-Htn5 also exhibits significant activity toward catechol oxidation [153].
Since Htn5 can effectively bind metal ions, conformation change upon each metal binding
can be expected. However, it has not been revealed how all the metal centers are involved
in the oxidative catalysis and in the antimicrobial activity of Htn5; and the correlation
between structure, antimicrobial activities, and metallo-Htn5 is unknown.
Ni
2þ
>
Zn
2þ
Search WWH ::

Custom Search