Chemistry Reference
In-Depth Information
[76,122]. It is widely used as a feed additive for livestock [123] and in “triple anti-
biotics” (along with polymyxin and neomycin) for human external use [124]. Bc con-
tains four
D
-amino acids, a thiazoline ring, and a cyclic heptapeptide structure formed
via a linkage between the sidechain amine of Lys6 and the C-terminal carboxylate of
Asn12 (
2
). It can inhibit metalloproteases presumably due to its metal-binding capabil-
ity [125], can inhibit a membrane-bound protein disulfide isomerase (PDI) [126], and
may serve as a selective inhibitor of b1andb7 integrin following a not yet known
mechanism [127]. However, it was recently shown to have only relatively minor effect
on PDI activity
in vitro
[128] which requires re-evaluation of Bc as a specific inhibitor
of PDI in cellular systems.
O
D-Orn YD-P eHis
D-Asp
Asn
12
Leu D-Glu
X
6
R
1
N
HN
ζ
O
Cys
2
S
NH
2
(
2
)
Bc requires a divalent metal ion for its activity [129] and can bind several divalent tran-
sition metal ions, including Co
2þ
,Ni
2þ
,Cu
2þ
,andZn
2þ
[130], and it triggers a slight
conformational change as discussed below [131]. Co
2þ
-Bc binds C
55
-isoprenyl (undecai-
soprenyl or bactoprenyl) pyrophosphate with a formation constant of 1.05
10
6
M
1
[132], which can prevent dephosphorylation of this lipid pyrophosphate to bind UDP sug-
ars for transport of the sugars during cell wall synthesis [133]. NMR study of Zn
2þ
-Bc
suggested that His-10 and the sulfur of thiazoline are coordinated to the metal [130]. EPR
study of Cu
2þ
-Bc indicated a tetragonally distorted Cu
2þ
center (g
x
¼
2.058, g
y
¼
2.047,
g
z
¼
534MHz) with coordinated thiazoline ring nitrogen, imidazole
of His10, and carboxylates of
D
-Glu4 and Asp11 [13b]. Extended X-ray absorption fine
structure (EXAFS) study of Zn
2þ
-Bc in solid form revealed three Ns and one O in the first
coordination sphere with a tetrahedral-like geometry [134], suggested them to be thiazo-
line nitrogen, His10 imidazole,
D
-Glu4, and possibly the N-terminal amino group. The
hyperfine-shifted
1
H NMR spectrum of Co
2þ
-Bc revealed the N
e
of His10, the carboxyl-
ate of
D
-Glu4, and the thiazoline nitrogen as the metal-binding ligands [131]. A structural
model of Co
2þ
-Bc built with relaxation times as distance constraints revealed a hydropho-
bic pocket formed by the side chains of Ile5 and
D
-Phe9 for possible binding with the
hydrocarbon chain of the sugar-carrying undecaisoprenyl pyrophosphate (Figure 1.12),
whereas the structure of apo-bacitracin from 2-D NMR showed that the side chains of
D
-
Phe9 and Ile8 are close to Leu3 [135]. Investigation of the Co
2þ
complexes of Bc conge-
ners, including the active Bc-B
1
and B
2
and the inactive stereo isomer A
2
and the oxidized
form F, revealed that proper metal binding is essential for Bc activity. The crystal struc-
ture of a Bc-bound serine protease complex shows an extended structure [136] which
would prevent metal binding. The conformational difference among Bc, its protease-
bound complex, metallo-Bc, and farnasylpyrophosphate-metallo-Bc complexes reflects
conformational flexibility of this peptide framework even for its seemingly rigid cyclic
structure.
2.261, and A
z(Cu)
¼








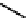











Search WWH ::

Custom Search