Chemistry Reference
In-Depth Information
Figure 8.11 Helicating ligands bearing di- or tripeptides as spacers and the thermo-
dynamically preferred titanium complex [14
3
Ti
2
]
4
of ligand 14 with parallel arrangement of
all three ligand strands.
For the preparation of well defined peptide helicates, metals with different coordination
preferences in combination with different ligand units can be used to gain some structural
control over the outcome of a coordination study.
The principle is schematically presented in Figure 8.12. If sequential ligands are used,
the strands can be orientated either parallel or antiparallel. The addition of one kind of
metal ion preferably results in an oligonuclear complex with an antiparallel orientation of
the ligand strands. The similar metal ions are approaching a coordination environment as
similar as possible. Nature tries to avoid charge separation between similar centers. If the
same (or a related) ligand strand reacts with two different kinds of metal ions, one will
prefer the “first”, the other the “second” coordination site. A parallel orientation of the
ligand strands will result in a heteronuclear complex [33].
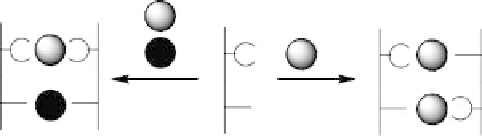
Figure 8.12 Control of the relative orientation (parallel versus antiparallel) of sequential lig-
ands (presented as straight line bearing two different binding sites for metals). They are able to
react with two different metals (grey and black) to result in a heterodinuclear complex with
parallel orientation of the ligands. Reaction with only one kind of metal (grey) results in the
formation of a complex with antiparallel orientation of the strands.

Search WWH ::

Custom Search