Chemistry Reference
In-Depth Information
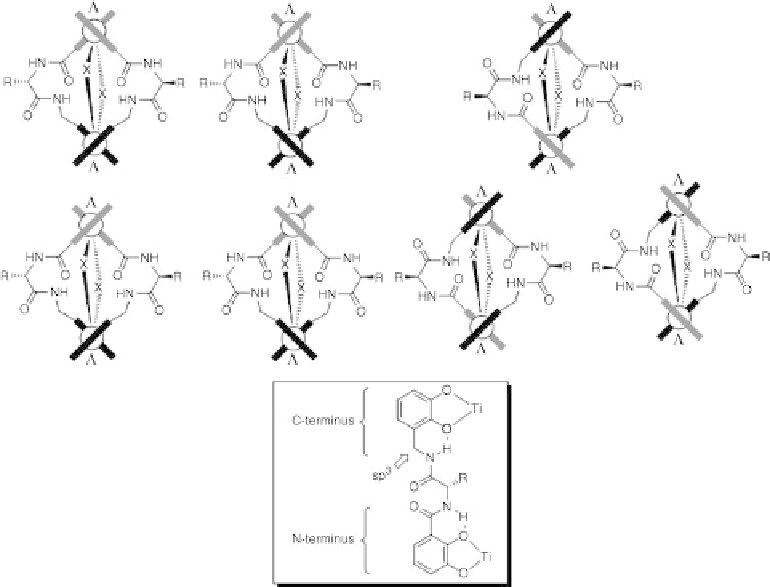
Figure 8.8 Schematic representation of the seven isomers which can be formed from ligands
13 and titanium(IV) ions. Catechol units are only shown as bars (grey: N-terminal; black: C-
terminal); X¼OR. The inset shows the preferred conformation of the ligand due to intra-
molecular NH-O hydrogen bonding.
control and the thermodynamically favored product is only obtained after a couple of days
at room temperature.
It was found that an initially high optical rotation of the solution of complexes
decreases over a period of some time. This effect indicates a “loss of stereochemical
information” which can be explainedbythepreferredformationofaLD heterochiral
dinuclear titanium(IV) complex. This assumption is supported by computational consid-
erations showing that four stabilizing intramolecular hydrogen bonds are present in the
heterochiral complex. In the corresponding homochiral compound only two can be
formed.
A series of crystal structures of Li
2
[(
13
)
2
(OR)
2
Ti
2
](R
Me, H) complexes with differ-
ent amino acids were obtained which allowed a conformational analysis. The final consid-
erations followed a series of sequential steps:
¼
1. From the observations of the optical rotation and from computational results it was
assumed that the dominating species adopts a heterochiral LD configuration at the
metal complexes (see above).
Search WWH ::

Custom Search