Chemistry Reference
In-Depth Information
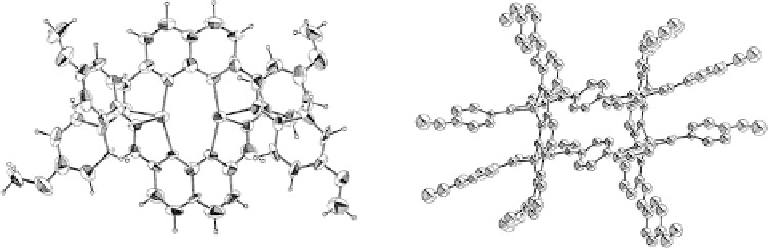
Figure 7.18 (a) ORTEP representations of the [Cu(9)
2
](ClO
4
)
2
complexes. (b) ORTEP repre-
sentations of the [Cu(11)]
4
(ClO
4
)
4
complexes, The hydrogen atoms are omitted for clarity and
50% probability ellipsoids are shown.
and ambidentate coordination modes of these kinds of Schiff-base ligands is shown here,
where the same ligand within the same complex adopts two different coordination modes,
Type X and Type XI (Figure 7.17).
7.3 Conclusions
General trends can be discerned from these studies of several series of metallomesogens
constructed from imino-oligopyridinic scaffoldings forming metallohelicates. While the
mesophase obtained with the Cu-terpy helicate is smectic, that of the corresponding bpy
complex is columnar and that obtained with a mononuclear copper(I) imino-pyridine
complex is columnar with hexagonal symmetry [72,73]. There are two main structural
differences between these polypyridine complexes that might account for this variation in
mesogenic morphology. First, the extended length of the central aromatic core is expected
to stretch the molecule into a calamitic shape that favors a smectogenic arrangement. Sec-
ond, the fluxional motion inherent to the terpy-based metallohelicate increases the
entropy of the system and favors the formation of the mesophase. It is this local fluctua-
tion that establishes microdomains sustaining liquid-crystalline behavior at room temper-
ature. Furthermore, the Cu-terpy helicate forms a highly unusual smectic mesophase
comprising layers of metallohelicates arranged in equidistant columns but without 3-D
correlation of the layers. Such structures are made possible by combining: (i) internal
flexibility of the coordinated polytopic ligands, (ii) ancillary coordination sites to stabilize
emerging redox centers, and (iii) multiple flexible sidechains.
The realization that such cationic complexes are liquid crystals at room temperature
opens the door to several broad and fruitful areas of supramolecular chemistry. With this
information at hand, it might be expected that the design and construction of nanoscale
molecular assemblies and supramolecular arrays intended to store, transfer or display
information could be reached in the near future. Although control of the supramolecular
structure is still a demanding challenge, the careful choice of molecular building blocks
makes it possible to predict the nature of the emergent supramolecular structure.
Search WWH ::

Custom Search