Chemistry Reference
In-Depth Information
R
1
R
1
R
2
R
2
O
O
R
3
R
3
O
O
N
N
O
N
N
Cu
Cu
N
N
O
R
3
N
N
O
R
3
O
R
2
R
2
R
1
O
R
1
OC
12
H
24
O
O
18
R
1
= R
2
= R
3
=
OC
12
H
24
O
19
R
1
= OC
12
H
25
; R
2
= R
3
=
O
OC
12
H
24
O
20
R
1
= R
2
= OC
12
H
25
; R
3
=
Figure 7.11 Schematic representation of the bipyridine based metallohelicate bearing vari-
ous numbers of acrylate fragments at the periphery.
unsuccessful due to severe auto-filtration of the light by the strongly absorbing helicates.
However, thermal polymerization using AIBN as initiator gave rise to a thermally stable
polymeric network in which the columnar organization of the helicate was maintained
(Figures 7.12 and 7.13). Close examination of the polymerized sample by X-ray diffrac-
tion revealed a mesomorphic state over a very large temperature range, as would be
expected for a rigidified structure. The diffraction pattern revealed a single narrow
Figure 7.12 Left hand side: photographs of the polymerized ligand precursor of helicate 20
(white polymer), duramer of the metallohelicate 20 (dark green), duramer obtained from the
dark green polymer after treatment with KCN in hot DMF. Right hand side: creme powder of
the non-polymerized ligand, precursor of metallohelicate 20 (non-mesophorphic ligand),
green powder metallohelicate 20 before polymerization (mesomorphic complex). The polym-
erized copper-helicate sits in the middle of the glass slides.



























































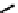


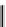






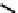

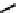































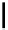






Search WWH ::

Custom Search