Chemistry Reference
In-Depth Information
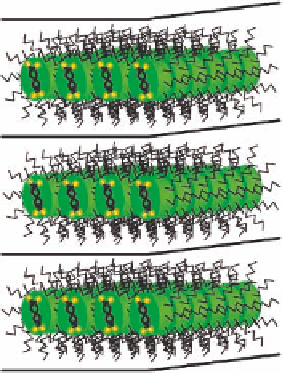
Figure 7.10 Idealized packing of the terpyridine based metallohelicate within the smectic
mesophase. The yellow signs represent the uncoordinated imine functions.
a viable method by which to obtain new types of self-organized assemblies of nano-
scopic dimensions.
Nevertheless, the stability and processability of metallomesogens is lower than that of
similar organic compounds and this drawback has restricted the development of these
materials. One strategy to improve their processability with the aim of fabricating smart
devices is the incorporation of metals into liquid-crystalline polymeric assemblies. The
design of such metallomesogenic polymers generally employs similar methodologies to
those used in organic liquid-crystalline polymers [65]. In the design of these reactive
mesogens, moieties that undergo radical polymerization (mainly acrylates or methacry-
lates) have generally been chosen because radical polymerization is more compatible
with organic functional groups than other polymerization techniques.
In situ
polymeriza-
tion of lyotropic metallomesogens has been studied in regard to biomembrane models,
drug-delivery systems, and templates for nanocomposites [66]. Along these lines a series
of reactive amphiphiles that contain transition metal or lanthanide ions chelated to car-
boxylate moieties were prepared and polymerized by subsequent irradiation in the pres-
ence of a radical photo-initiator. Selection of an appropriate metal ion enables both the
dimensions and the properties of the nanostructured polymeric network to be controlled
[67]. The confinement of metal ions within the nanometric channels of the polymeric
matrix provides the possibility of using these materials as nanoreactors for catalytic trans-
formations [68]. Also interesting is the generation of CdS nanoparticles in nanometric
channels by exposing a polymerized network of the Cd
II
metallomesogen to H
2
S vapor
[69]. More recently
in situ
polymerization of thermotropic metallomesogens has proven
to be feasible, affording anisotropic materials for application in optical technologies [70].
Bipy-Schiff base metallohelicates bearing between four and 12 acrylate substituents
form self-organized structures, some of which are shown in Figure 7.11. In all cases, the
material remains mesomorphic at temperatures between
15 and 140
C as deduced from
differential scanning calorimetry (DSC). Photochemically initiated polymerization was
Search WWH ::

Custom Search