Chemistry Reference
In-Depth Information
H
3
CO
N
N
N
N
N
12
OCH
3
OCH
3
H
3
CO
N
N
N
N
Cu
Cu
N
N
N
N
N
N
H
3
CO
13
OCH
3
Figure 7.5 Schematic representation of the imino-terpyridine ligands and its corresponding
copper-helicate.
auspicious for some electronic interactions as well as for coordination to a metal in a
higher oxidation state (Figure 7.6) [46].
Redox cycling in these segmented copper helicates is effective and the helicate is stable
in different oxidation states of the metal and also for successive reduction of the ligands.
Such stability is derived from the ability of the ligand to provide both 4- and 5-coordinate
geometries around the metal centers. These requirements are essential if the metalloheli-
cate is to be used as a redox catalyst or artificial enzyme. There appears to be a limited
number of examples of copper helicates or other such related structures that do not
undergo large-scale structural reorganization following oxido-reduction reactions.
Figure 7.6 ORTEP drawings of the [Cu
2
(12)
2
](ClO
4
)
2
complexes, showing 50% probability
ellipsoids. The hydrogen atoms are omitted for clarity.








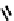
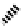
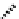






















































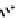
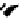












Search WWH ::

Custom Search