Chemistry Reference
In-Depth Information
7.2 Imino-Bipyridine and Imino-Phenanthroline Helicates
These metal ion-induced, self-assembled structures were first found with an arene-chro-
mium Schiff-base ligand constructed from arene-chromium
ortho
-toluidine subunits (Fig-
ure 7.2) [34]. The high stability constants for the Cu(I) complexes were determined by
spectrophotometric titrations that were analyzed in terms of a multistep process. An
important caveat of these studies is that positive cooperativity is not mandatory for the
formation of a helicate. When reacted with Cu(I), both di-imino-bipyridine and -phenan-
throline ligands were found to provide a mixture of 1 : 1 and 1 : 2 (M:L) complexes, the
deep violet 1 : 1 species in fact being shown by mass spectrometry to be 2 : 2 complexes
to which, for the bipyridine derivative, a helicate structure (Figure 7.2) was assigned. The
bipyridine moiety acts as a bridge rather than a chelate and the chelating centres are pro-
vided by the imine (
Im
N
) and pyridine (
Py
N
) donors, with each metal ion coordinated by
two of these fragments.
It was surmised at that time that the binuclear complexes were the kinetic products of
complexation (formed rapidly under non-equilibrating conditions in non-polar solvents),
whereas the mononuclear complexes are the thermodynamic product of complexation
when prepared under equilibrating conditions in a coordinative solvent such as acetoni-
trile [34].
These studies gave rise to interesting questions concerning the possible involvement of
an electronic effect inherent to the strong withdrawing character of an arene-chromium
tricarbonyl moiety and of possible steric crowding around the potential
Py
N
,
Py
N
chelating
center versus the
Py
N
,
Im
N
chelating fragment. It motivated a research program aimed at
the construction of imino-oligopyridine frameworks from fluoroaniline, anisidine, and
functionalized anilines. A palette of the resulting ligands is given in Figure 7.3 and syn-
thetic details, purification procedures, and characterization may be found in the cited liter-
ature. These ligands were synthesized by a standard protocol for Schiff-base synthesis
OC
OC
CO
Cr
CO
OC
CO
Cr
N
N
N
N
N
Cr
OC
CO
CO
N
1
3
OC
OC
OC
CO
CO
N
OC
Cr
Cr
N
N
N
N
N
Cu
Cu
N
N
N
N
Cr
Cr
CO
OC
OC
Cr
OC
CO
CO
OC
OC
2
CO
Figure 7.2 Arene-chromium imino-bipyridine and imino-phenanthroline ligands and their
copper(I) metallohelicate complexes. The charges have been omitted for the sake of clarity.


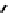
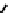
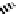

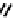







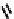
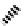

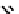




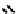

















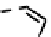




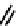





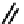


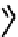


























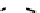
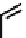

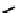



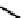
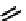





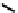

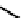



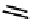






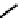

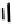



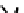

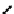







Search WWH ::

Custom Search