Chemistry Reference
In-Depth Information
tetrakis(3-heptafluoro-butylryl-(
þ
)-camphorato)europium displays a record
g
lum
¼þ
1.35
10
2
for organic molecules, including
helicenes. One drawback of CPL resides in the weakness of the signals but substantial
improvements are being made, particularly with respect to the excitation wavelength used
for Eu
III
, and instrumentation can be expanded to use TRD so that CPL may develop as an
essential tool for the enantiomeric recognition of biological substrates [114].
There are not many reports on optically active lanthanoid helicates. The first one is
concerned with a Nd
III
helicate with the
R,R
isomer of (L12b)
2
(Scheme 6.2),
[Nd
2
(L12c)
3
]. The corresponding f-f transitions are optically active indicating that the
chiral linking group induces chirality at the metal centre. Molecular dynamic calculations
suggest a D,D configuration [51].
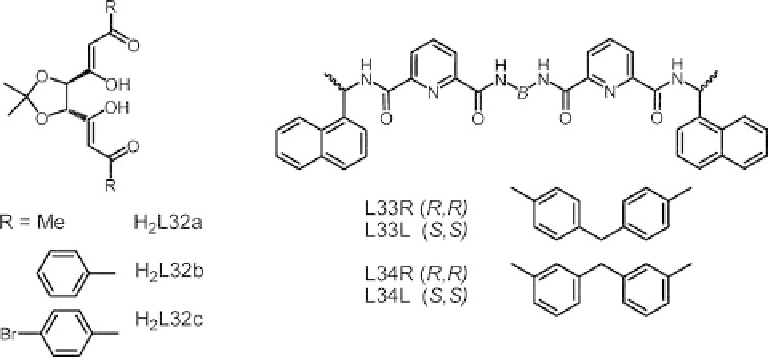
Examples of f-f helicates are also documented with ligands H
2
L32a-c derived from
R,
R
-tartaric acid (Scheme 6.7). Both the neutral triple-stranded [Ln
2
(L32a-c)
3
] and cationic
quadruple-stranded [Eu
2
(L32c)
4
]
2þ
helicates display CD spectra in dichloromethane
which arise from the ligand. Luminescence from Eu
III
,Tb
III
and, to a lesser extent, Ho
III
is sensitized but no CPL spectra are reported [116].
T. Gunnlaugsson and collaborators have succeeded in getting enantiomerically pure f-f
homometallic triple-stranded helicates by f-directed synthesis with ligands L33 [117] and
L34 [118]. Pairs of
R,R
-[Ln
2
(L)
3
]
6þ
and
S,S
-[Ln
2
(L)
3
]
6þ
helicates have identical NMR
spectra, which proves their appearance as pairs of enantiomers as confirmed by the corre-
sponding CD spectra. Luminescence of Eu
III
10
3
[115]. This is to be compared with
to
is somewhat sensitized (quantum yields
<
1%) and the overall emission spectra of the two enantiomers are identical and diagnos-
tic for
C
3
symmetry while on the NMR time scale the averaged symmetry of the self-
assembled edifices appears to be
D
3
. But their CPL spectra are opposite, which enabled
the authors to established the absolute configuration as being L,L for [Eu
2
(L33R)
3
]
6þ
and
D,D for [Eu
2
(L33S)
3
]
6þ
(Figure 6.20).
Isolation of pure chiral nd-4f helicates was achieved by C. Piguet
et al.
who took
advantage of the inertness of Cr
III
in [LnCr(L25)
3
]
6þ
(Scheme 6.6) [119]. Indeed, the
Scheme 6.7 Chiral ligands for the self-assembly of lanthanoid helicates.
Search WWH ::

Custom Search