Chemistry Reference
In-Depth Information
n
þ
Table 6.13 Lifetimes and energy transfer yields in microcrystalline [MLn(L25)
3
]
helicates
at 10 K (first number) and 295 K (second number) under ligand excitation [90].
LnM
a
t
(
5
D
0
) (ms)
t
(
2
E) (ms)
h
et
(LnCr) (%)
GdCr (1)
3.66(3), 0.29(1)
EuZn (2)
2.53(1), 1.67(2)
EuCr (3)
0.55(4), 0.59(1)
3.46(1), 0.09(1)
78(5), 65(3)
EuZn (4)
2.19(1), 1.98(1)
EuCr (5)
0.75(1), 0.66(1)
3.12(1), 0.05(1)
66(2), 67(2)
b
TbCr (6)
3.39(1), 0.17(1)
100, 100
a
(1)
¼
[GdCr(L25)
3
](CF
3
SO
3
)
6
(H
2
O)
6
; ( )
¼
[EuZn(L25)
3
]ClO
4
(CF
3
SO
3
)
4
(MeCN)
4
; ( )
¼
[EuCr(L25)
3
]
(CF
3
SO
3
)
6
(MeCN)
4
;(4)
¼
[EuZn(L25)
3
](ClO
4
)
5
(H
2
O)
2
;(5)
¼
[EuCr(L25)
3
](CF
3
SO
3
)
6
(H
2
O)
4
;(6)
¼
[TbCr
(L25)
3
](CF
3
SO
3
)
6
(H
2
O)
3
.
b
No Tb emission.
6.3.3 Control of f-Metal Ion Properties by d-Transition Metal Ions
Heterometallic d-f molecular helicates are appealing because electronic communication
between metal ions along the molecular axis can be induced and controlled, which repre-
sents an additional tool in the hands of synthetic chemists for tuning optical and/or mag-
netic properties. With respect to luminescence, motivations for resorting to d-metal
containing chromophores are to shift the excitation wavelength from the UV to the visible
range, which is particularly appreciated when dealing with biological systems, to lengthen
the apparent lifetime of the Ln
III
emitting ion and to efficiently transfer energy onto the
lanthanoid ion.
6.3.3.1 Switching Eu
III
Luminescence On and Off
Control of Eu
III
luminescence is exemplified in EuFe
II
helicates by simple modifica-
tion of the ligand. For instance, if a methyl group is introduced in the 5-position of
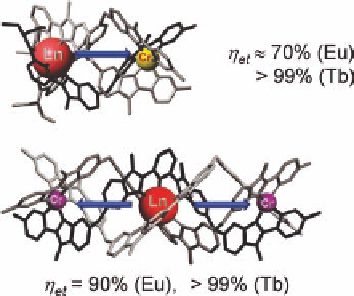
Figure 6.15 Di- and trinuclear triple-stranded Cr
III
-Ln
III
helicates experiencing intra-
molecular axial energy transfers.
Redrawn from crystallographic data published in Refs.
[11,90].




Search WWH ::

Custom Search