Chemistry Reference
In-Depth Information
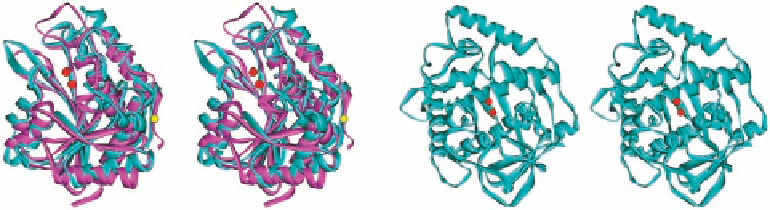
Figure 1.8 Left: stereo view of superimposed sAP (lavender; PDB ID 1CP7) and aAP (cyan;
PDB 1AMP), with the active-site di-Zn in red sphere and Ca (in sAP) in yellow. Right: stereo
view of di-Cu catechol oxidase from sweet potato, showing the very different folding from that
of sAP. The two Cu ions are shown in red.
catalysis, despite its very similar folding and active-site coordination (Asp/His on one
metal, Glu/His on the other metal, and a bridging Asp) to those of aAP (Figure 1.8),
which indicates that the microenvironment such as the proximal amino acid residues
around the active-site coordination sphere must be more significant than the folding of
the peptide backbone for proper functions and specific activities.
Streptomyces
AP is a di-Zn
2þ
-containing 30-kDa enzyme which consists of a central
b-sheet core surrounded by helices with the active site found within the b-sheet region
(Figure 1.8), wherein the dinuclear site can selectively bind metal ions such as Co
2þ
and
Mn
2þ
in the two metal-binding sites of sAP [52]. The various metal derivatives exhibit
significant alternative catalysis toward a phosphodiester substrate, despite the fact the lat-
ter frequently serves as a transition-state inhibitor, which is not the case for aAP [53].
Moreover, the di-Cu
2þ
derivative of the enzyme shows significant activity toward cate-
chol oxidation [54], despite its protein folding and active-site coordination environment
being completely different from those of catechol oxidase with three His residues bound
to each Cu (Figure 1.8). The observations presented herein indicate that protein folding in
not the only control for showing specific enzyme catalysis (i.e., sAP vs aAP toward alter-
native catalysis) and the folding and/or active-site coordination sphere does not need to be
restricted to a certain pattern to exhibit a specific catalysis (i.e., di-Cu-sAP vs catechol
oxidase).
1.2.4 Protein Misfolding: Causes and Implications - Cu, Zn-Superoxide Dismutase
The superoxide dismutase (SOD) family has four distinct groups, that is, Cu-, Zn-, Fe-,
Mn-, and Ni-containing, which are responsible for catalyzing the conversion of super-
oxide anionic radical to O
2
and H
2
O
2
to protect the cellular environment from damage by
superoxides generated during respiration or through the oxidative activity of immune cells
[55]. The Cu,Zn-containing SOD (SOD1) is a dimeric protein, with each monomer con-
sisting of an eight-stranded b-barrel and electrostatic and metal-binding loops (Figure 1.9)
[56]. The electrostatic loop features Arg143 for hydrogen bonding to superoxide and
Thr137 in conjunction with Arg143 to limit the anions coming into the copper-active site.
The catalytic site features a unique bridging His63 residue between the two metal ions at
Search WWH ::

Custom Search