Chemistry Reference
In-Depth Information
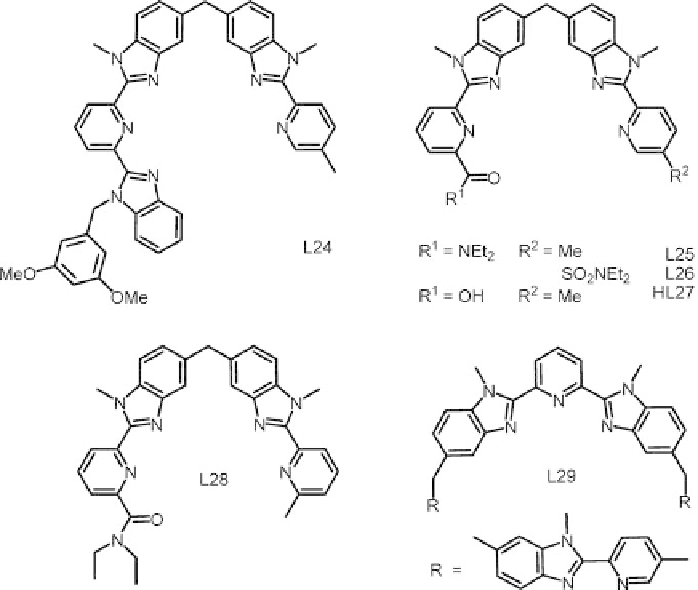
Scheme 6.6
Ligand for assembling bimetallic 4f-nd helicates.
listed in Table 6.4 amount to 17 236, 17 235 and 17 231 cm
1
for N
9
,N
6
O
3
(amide) and
N
6
O
3
(carboxylate) environments, respectively. Experimental data are in good agreement
for L24 but somewhat lower than predicted for L25 and L26 (17 224-17 229 cm
1
), possi-
bly due to the large spin delocalization evidenced by NMR and causing a larger nephe-
lauxetic effect. In contrast, the experimental nephelauxetic effect for HL27 is 5 cm
1
smaller than the calculated one.
All
7
F
J
transitions can be analyzed on the basis of a distorted
D
3
symmetry as
seen from the splitting of the
7
F
1
level into two sublevels labelled A
2
and E. There are,
however large differences between the various helicates and, also, between solid state and
solution samples, particularly in the case of L26. Looking at the D
E
(A
2
5
D
0
!
E) energy differ-
ence for the Zn
II
helicates, which is directly proportional to the
B
0
ligand-field parameter
[23], the strength of the ligand field induced by the various ligands at low temperature
increases in the series L45 (93 cm
1
) <L26 (118) <L25 (127) <HL27 (138) <L28 (146).
This can be understood when considering the inner sphere composition: a N
9
environment
generates a weaker field than a N
6
O
3
one. The weaker field induced by L26 with respect to
L25 arises from the less distorted coordination polyhedron in [EuZn(L26)
3
]
5þ
compared
with [EuZn(L25)
3
]
5þ
while the largest field observed for the helicate with L28, with
respect to the carboxylic acid HL27, results from the weaker coordination of the 3d transi-
tion metal, allowing a tighter wrapping of the ligand strands around the lanthanoid ion.
Search WWH ::

Custom Search