Chemistry Reference
In-Depth Information
Eu(N
6
O
3
)Eu(N
9
)
E
an
= 16 883 cm
-1
E
an
= 16 949 cm
-1
17.20
17.20
17.22
17.22
17.24
17.24
17.26
17.26
E
/ 10
3
cm
-1
5
D
0
7
F
j
4
2
j
= 1
Eu(N
6
O
3
)
E
E
exc
= 17 211 cm
-1
17 211
1
3
Eu(N
9
)
E
exc
= 17 238 cm
-1
14.0
14.5
15.0
15.5
16.0
16.5
17.0
17.5
E
/10 cm
E
/10
3
cm
-1
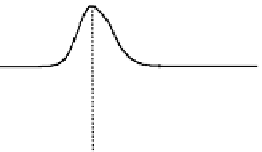
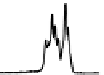
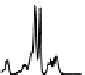
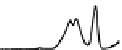
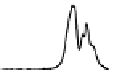
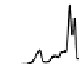
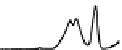
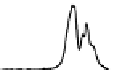
Figure 6.13 Top: excitation profiles of the
5
D
0
7
F
0
transition of [Eu
3
(L22)
3
]
9þ
obtained by
selectively monitoring components of the
5
D
0
!
7
F
1
transition. Bottom: corresponding emis-
sion spectra under selective excitation of the
5
D
0
7
F
0
transitions. Solid state sample at 10 K.
Redrawn with permission from [86]. Copyright 2003 WILEY-VCH Verlag GmbH & Co. KGaA,
Weinheim.
respectively, evidencing a somewhat larger distortion from the idealized
D
3
symmetry for
the latter site.
The overall quantum yield in acetonitrile amounts to only 0.3%, that is about 30 times
less than the one of the reference helicate [Eu
2
(L4)
3
]
6þ
with N
6
O
3
coordination sites
(emission from the central site contributes little to the overall luminescence). The
5
D
0
ð
and
5
D
0
ð
lifetimes are the same at 10 K, 2.3(1) and 2.2(1) ms and decrease
to 1.7ms at room temperature. Interestingly the temperature dependence of
N
6
O
3
Þ
N
9
Þ
(N
9
) is less
pronounced than for [Eu
2
(L3)
3
]
6þ
, suggesting that the slide of the ligand strands around
the N
9
site shifts the LMCT at slightly higher energy. However, the much lower than
expected quantum yield probably reflects an intramolecular quenching of the Eu(N
6
O
3
)
luminescence from this state located on the neighbouring EuN
9
moiety [86].
Heterometallic 4f-4f
0
-4f helicates have also been obtained in solution and the affinity of
the two sites for a range of Ln
III
t
La, Nd, Sm, Eu, Yb, Lu, Y) has been deter-
mined. In particular a La-Eu compound could be isolated and crystallized (Figure 6.14).
One important question was to determine if the crystals were homogeneous or contained
different species. Chemical elemental analysis corresponds to [La
0.93
Eu
2.07
(L22)
3
]
(CF
3
SO
3
)
9
, while the formula that can be deduced from single-crystal X-ray crystallogra-
phy is [La
0.96
Eu
2.04
(L22)
3
](CF
3
SO
3
)
9
. Refinement of the diffraction data leads to the
ions (Ln
¼




























Search WWH ::

Custom Search