Chemistry Reference
In-Depth Information
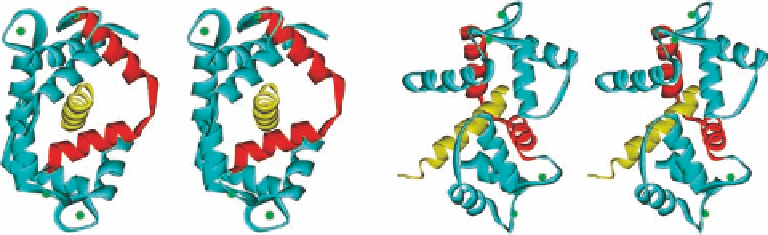
Figure 1.6 Two different views (rotating by 90
)ofCa
4
-calmodulin-bound “IQ motif” of
the Cav1.1 channel (PDB ID 2VAY). The interdomain helix is colored in red, the IQ motif in
yellow, and the four Ca
2þ
ions as green spheres.
resulting in conformational changes and interaction with other proteins and enzymes to
perform its regulatory role (Figure 1.5) [36]. Ca
2þ
binding to the C-terminal sites stabilizes
the long interdomain helix via a Tyr138-Glu82 interaction, which in turn disrupts two
interactionhelicesbybreakinganAsp/Glu2-Lys77interaction,whichisfollowedbyCa
2þ
binding to the N-terminal sites to form a binding cleft for target proteins [37].
Calmodulin can bind various molecules, including drugs, peptides, and their regulating
target proteins. Calmodulin-modulated Ca
2þ
signaling is thus attributed to the different
responses of these target molecules to the conformational change of calmodulin upon
Ca
2þ
binding. The difference in Ca
2þ
binding and target interactions of the two lobes
also enable calmodulin to work out local and global Ca
2þ
sensing and signaling through
conformational change [38]. Further, the binding of target molecule to calmodulin can
also influence the Ca
2þ
sensitivity of calmodulin [39]. The calmodulin-binding regions in
the target proteins are comprised of short helical segments of
14-26 amino acids with a
high occurrence of hydrophobic and basic residues for high affinity and specificity with-
out the need for sequence specificity [40]. Such dramatic conformational change is illus-
trated in Figure 1.6 for Ca
4
-calmodulin binding binding to the “IQ” “motif” in the a
1
subunit of the L-type voltage-dependent Ca
2þ
channel Cav1.1, which undergoes Ca
2þ
-
and CaM-dependent channel facilitation and inactivation [41]. The C-terminal conforma-
tion of the a
1
subunit is critical for channel function and has been proposed to regulate the
gating machinery of the channel [42]. The binding causes a significant conformational
change in calmodulin, especially the kink at positions 79-81 of the interdomain helix,
which results in wrapping around the peptide (Figure 1.6). Taken together, calmodulin
represents one of the best examples showing significant metal- and ligand-induced con-
formational changes.
1.2.3.2 Carboxypeptidase A Catalytic Mechanism
Another example of dramatic conformational changes in a metalloenzyme is well repre-
sented in the action of carboxypeptidase A, a pancreatic proteolytic enzyme. It belongs to
a family of exopeptidases responsible for catalyzing the hydrolysis of peptide bonds at the
C-terminus of peptides and proteins. It plays a regulatory role or complements the action
Search WWH ::

Custom Search