Chemistry Reference
In-Depth Information
1.2.2.3 Metallothionein and Heavy Metal Regulation
The concentration of zinc must be well controlled in the cell, as high concentrations can
be toxic and cause mitochondrial dysfunction. Metallothionein plays a central role in zinc
homeostasis since it is the major protein important in regulation of the zinc level in the
cell and its translocation [20] and it has been shown to induce an effect on brain neurons
by binding to neuronal receptors and initiating pathways which cause neurite survival
[21,22]. Metallothionein regulates the flow of zinc and copper in the cell and can further
prevent poisoning from exposure to toxic cadmium and mercury. Its overall role is sug-
gested to be the control of the distribution of zinc as a function of the cellular energy state
and it has been implicated in the following functions [23,24]: (a) intracellular zinc trans-
port, (b) zinc binding and exchange (e.g., with the zinc cluster protein Gal4, zinc finger
transcription factors such as TFIIIA, and aconitase) as well as a zinc-specific chaperone,
(c) oxidoreductive properties of cysteine bound zinc as cysteine ligands are redox-
sensitive regulatory switches [25], (d) controlling cellular zinc distribution as a function
of the energy state of the cell as shown by the interaction with ATP, GSH, and ROS and
zinc distribution to enzymes in metabolic networks of gene expression and respiration
[26], and (e) a possible role in neural activity, storing and distributing zinc for the neuro-
nal network and protecting it against cellular damage as well as neuronal recovery
through binding to neuronal receptors initiating signal transduction pathways [22].
One function of metallothionein may resemble that of the iron-storage protein ferritin in
terms of its zinc-storage capability. It is a small protein rich in cysteine (20 cysteines in a
total of 62 amino acids in human metallothioneine), but without aromatic amino acids such
as tyrosine or histidine. The apo protein can bind a total of seven equivalents of divalent
metal ions with
d
10
configuration such as Zn
2þ
or Cd
2þ
in two noninteracting domains
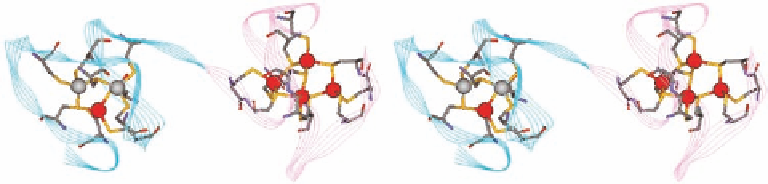
(Figure 1.4) [27] and up to six Cu
þ
ions in each domain [28]. The protein can bind up to
seven Zn
2þ
or Cd
2þ
ions in tetrahedral coordination spheres, or 12 three-coordinate Cu
þ
ions with only cysteine residues. The binding of metal converts the random coil conforma-
tion of apo metallothionein into a folded two-domain structure (Figure 1.4). Cd
2þ
binding
has been extensively studied with C-13 NMR [29], wherein structural flexibility was
observed. The absence of hydrophobic residues for stabilizing a folded form is compen-
sated by the presence of the metal-thiolate core in the folding of this protein.
Apo metallothionein retains the backbone conformation imposed by the formation of
the metal-thiolate clusters. REF computational studies indicate a potential H-bonding
Figure 1.4 Stereo view of the structure (PDB 4MT2) of Cd
5
Zn
2
, showing the N- (cyan) and C-
(pink) domains with the coordinated Cys residue.
Search WWH ::

Custom Search