Chemistry Reference
In-Depth Information
NH
2
S
M
N
O
O
M(OAc)
2
NH
2
M
S
SH
2
14
:M=Ni
15
:M=Zn
M
N
S
2
16
:M=Ni
17
:M=Zn
Scheme 5.7 Synthesis of complexes 14 and 15 followed by subcomponent self-assembly to
the dinuclear complexes 16 and 17.
We found that nickel(II) and zinc(II) are excellent templates for this type of chemistry, as
indicated by the facile synthesis and stability of the 2-thiolatobenzaldehyde complexes
14
and
15
(Scheme 5.7) [56]. The molecular structure of the square-planar nickel complex
14
has been determined, showing a
cis
-configuration of the oxygen donors. The zinc complex
15
most likely possesses a tetrahedral coordination geometry. These features make com-
plexes
14
and
15
ideal building blocks for a subsequent subcomponent self-assembly
reaction via a double Schiff base condensation, leading to the dinuclear complexes of type
[M
2
L
2
](
16
:M
Zn) where L is a bis(bidentate) N,S^N,S ligand.
In fact, reaction of the nickel complex
14
with 4,4
0
-diaminodiphenyl-methane afforded
[Ni
2
(N,S^N,S)
2
]
16
as a brown solid. The NMR spectra of complex
16
showed only sig-
nals for half of each ligand strand, indicating the formation of a highly symmetric com-
pound. The molecular structure of
16
was unequivocally established by an X-ray crystal
structure determination (Figure 5.19, left) which revealed the formation of a dinuclear
double-stranded nickel helicate. The two nickel centers in
16
are each coordinated by two
thiolato-imine units in a nearly perfect square-planar geometry. Both six-membered
C
3
NSNi rings are significantly bent along the N
¼
Ni;
17
:M
¼
S vector, placing the parent phenyl
groups on opposing sides of the plane defined by the four donor atoms (see the coordina-
tion environment for the bottom nickel atom in Figure 5.19, left). The ligand strands are
wrapped around the NiNi axis in a helical manner with a twist angle of 159.5
.
Interestingly, the subcomponent self-assembly of zinc precursor
15
with 4,4
0
-diamino-
diphenylmethane proceeds more readily, enabling an efficient one-pot synthesis of
17
at
ambient temperature (Scheme 5.7).
In situ
preparation of zinc complex
15
from
o
-mer-
captobenzaldehyde and zinc acetate was followed by the addition of 4,4
0
-diaminodiphe-
nylmethane to generate [Zn
2
(N,S^N,S)
2
]
17
as a yellow solid. In addition to the expected
signals for a dinuclear double-stranded zinc helicate, a second set of signals with lower


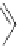








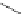
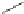

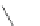
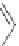
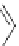


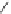




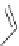






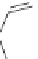










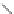

















Search WWH ::

Custom Search