Chemistry Reference
In-Depth Information
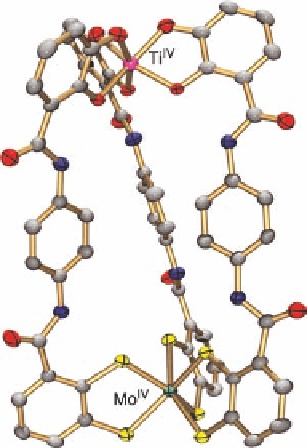
Molecular structure of anion L,L-[TiMo(13)
3
]
4
.
Figure 5.17
studied. The reaction of three equivalents of H
4
-
13
with one equivalent of both [TiO
(acac)
2
] and [MoCl
4
(CH
3
CN)
2
] in the presence of Li
2
CO
3
and Na
2
CO
3
gives a dark green
solution. It was assumed that the presence of two different donor groups in the ligand aids
in the selective formation of the heterobimetallic complex [TiMo(
13
)
3
]
4
.
The addition of four equivalents of (PNP)Cl to the methanolic solution yields, after 12 h,
a dark green precipitate of (PNP)
4
[TiMo(
13
)
3
] which is only soluble in DMF. Crystals of
Li
0.5
(PNP)
3.5
[TiMo(
13
)
3
]
H
2
O have been obtained by vapor diffusion of
diethyl ether into a solution of (PNP)
4
[TiMo(
13
)
3
] in DMF [50]. The (PNP)
4
[TiMo(
13
)
3
]
used for the crystallization experiments was found to contain some traces of lithium cat-
ions, which were not detected by NMR spectroscopy. Structure analysis (Figure 5.17)
revealed that the different binding preferences of the donor groups in
13
4
for the two
different metal ions indeed leads to a triple-standed heterodinuclear complex with parallel
orientation of the ligand strands. The complex tetraanion [TiMo(
13
)
3
]
4
contains distorted
OC
{Ti(cat)
3
}(
4.5DMF
1.5EtOH
40.4
) and distorted
TP
{Mo(bdt)
3
} polyhedra (
23.8
). The helical
f
¼
f
¼
Ti 12.423(15) A
]in[TiMo
(
13
)
3
]
4
measures 42.6
. Since the compound crystallized in a centrosymmetric space
group, the D,D-enantiomer is also present in the crystal lattice.
In the solid state, two enantiomeric helicates, L,L-[TiMo(
13
)
3
]
4
and D,D-[TiMo
(
13
)
3
]
4
, are connected via a PNP
þ
cation which resides on a crystallographic inversion
center. This leads to a tetranuclear unit {[TiMo(
13
)
3
]
twist between the two L,L-configurated metal centers [Mo
[TiMo(
13
)
3
]}
7
(Figure
5.18, left). The complete formula for this assembly is {(PNP)
3
[TiMo(
13
)
3
]
(PNP)
[TiMo(
13
)
3
](PNP)
3
}
. The lithium cation missing for charge neutrality could not be
located by X-ray crystallography.
The importance of alkali metal cations for the stereoselective self-organization of dinu-
clear triple-stranded helicates has been described several times [5c,d,f,h,40a]. The
(PNP)
Search WWH ::

Custom Search