Chemistry Reference
In-Depth Information
SH
SH
SH
SH
SH
SH
SH
SH
HN
O
O
O
O
HN
NH
NH
NH
NH
HN
O
O
O
HN
O
OH
OH
OH
OH
OH
OH
OH
OH
H
4
-
10
H
4
-
11
H
4
-
12
H
4
-
13
Figure 5.4 Polydentate benzene-
o
-dithiol/catechol ligands.
The rigid ligand backbone of complex (NEt
4
)[Co(
14
)] probably causes the limited sta-
bility of this complex since it does not permit a strain-free square-planar coordination
geometry at the metal center. In addition, the halophilicity of the titanium atoms also
determine the stability of (NEt
4
)[Co(
14
)]. The reaction of [(Cp
2
Ti)
2
(
14
)] with a source of
chloride ion like NMe
4
Cl yielded complex (NMe
4
)[Ti(Cp)(
14
)] (Figure 5.5) [31]. The
reaction is most likely initiated by the substitution of at least one benzene-
o
-dithiolato
group from one titanium center by chloride ions. Several reaction pathways are conceiv-
able for the following substitution of a cyclopentadienyl group at the other titanium center
by the liberated benzene-
o
-dithiolato donor [32] and the formal elimination of unstable
[Ti(Cp)
3
Cl]. In contrast to the situation in the strained square-planar anion [Co(
14
)]
, the
two benzene-
o
-dithiolato donors in [Ti(Cp)(
14
)]
occupy the basal plane of a square-
pyramidal complex anion and are bent into an
exo
/
endo
conformation relative to the
cyclopentadienyl ligand. Probably due to the steric requirements of the phenylene
S
SH
TiCp
2
(NEt
4
)
SH
S
R
HN
NH
O
O
O
O
Cp
2
TiCl
2
NEt
3
1. (NEt
4
)
2
[CoCl
2
]
2. O
2
NH
NH
S
S
Co
R
R
-Cp
2
TiCl
2
S
S
HCl
NH
NH
O
O
R = CH
2
C
6
H
4
CH
2
: (NEt
4
)[Co(
14
)]
R = CH
2
(CH
2
)
5
CH
2
: (NEt
4
)[Co(
15
)]
S
SH
TiCp
2
S
SH
R = CH
2
C
6
H
4
CH
2
: [(Cp
2
Ti)
2
(
14
)]
R = CH
2
(CH
2
)
5
CH
2
: [(Cp
2
Ti)
2
(
15
)]
R = CH
2
C
6
H
4
CH
2
: H
4
-
14
R = CH
2
(CH
2
)
5
CH
2
: H
4
-
15
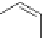
Scheme 5.2 Synthesis of di(titanocene) complexes of ligands 14
4
and 15
4
and ligand
transfer to give the Co
III
complexes.






























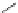












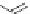

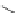


































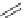



























































Search WWH ::

Custom Search