Chemistry Reference
In-Depth Information
Figure 1.1 Crystal structure of zinc finger-DNA complex (of Zif268). The Zn(II) ions (black
spheres) in the three “fingers” (cyan) are bound to the protein through two Cys and two His
residues and hold the a-helical/ b-sheet structural motifs together.
into the major groove of DNA (Figure 1.1; PDB 1ZAA) [7], representing one typical exam-
ple of metal- and ligand-mediated conformational change of a natural metallofoldamer.
1.2.2 Metal-Triggered Conformational Change of Proteins
1.2.2.1 Cytochrome c and Heme Binding
The cytochrome c family of electron-transfer proteins has a high a-helix content and a
heme cofactor that is postranslationally modified to covalently attach to the protein by
two thioether bonds between the vinyl group of heme and two cysteine residues within
the motif Cys-Xaa-Xaa-Cys-His [8]. The folding of cytochrome c has been studied by
proton to deuterium exchange equilibrium monitored with NMR [9]. The folding
sequences involve the interaction of the two terminal helices (N- and C-, respectively)
first, followed by joining of the 60s helix. The heme center locks in the designated pocket
and this is followed by final coordination of the Met80 in the sixth position. These three
helices containing the axial methionine appear to be the minimal structural requirement in
cytochrome c folding (Figure 1.2) [10]. The axial heme ligand is not conserved among all
the proteins and can be found to be histidine, asparagine, or it can be absent [11]. The
cytochrome c family has also been implicated in the apoptotic mechanism of cell death
Figure 1.2 Comparison of the three-dimensional structures of two different cytochromes c.
Left: crystal structure of tuna cytochrome c (PDB ID 3CYT). Right: solution structure of oxi-
dized cytochrome c from
Bacillus pasteurii
determined by NMR. The three helices are in
cyan, the extra loop in the tuna enzyme is shown in lavender, the heme is shown in red.

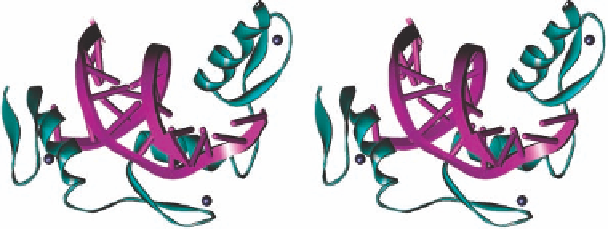
Search WWH ::

Custom Search