Chemistry Reference
In-Depth Information
Br
Br
Br
Ga(NO
3
)
3
or FeCl
3
OH
O
O
M
Li
LiClO
4
))))
O
O
O
M
O
O
O
O
O
O
ClO
4
M
O
O
O
M
OH
O
O
Br
Br
Br
3
3
67
[M
2
(
67
)
3
]
[LiM
2
(
67
)
3
]ClO
4
Figure 4.30 Tartaric acid-derived bis(b-diketonate) ligand 67, its triple-stranded dinuclear
gallium(III) or iron(III)helicates, and their use as metalla-cryptand for the complexation of lith-
ium ions.
4.7.9 Catecholate Ligands and Other Dianionic Ligand Units
Besides 2,2
0
-bipyridines, catechol ligands are probably the most often used metal binding
motifs in helicate chemistry. Therefore, it does not come as a surprise that enantiomeri-
cally pure bis(catechol) ligands have also been prepared and tested with regard to their
ability to undergo diastereoselective self-assembly to triple-stranded helicates upon coor-
dination to suitable metal ions, like gallium(III) or titanium(IV) ions. Again, both strate-
gies - either using a chiral building block as the centre between the two catechol units, or
putting it in the outer periphery - have been successfully applied. T. D.P. Stack, for
instance, prepared chiral ligands in which he used chiral diamines in order to link two
catechol groups via amide bonds [70]. Interestingly, he found that ligands with sterically
constrained C
2
-linkers between the amide groups gave rise to tetranuclear clusters rather
than dinuclear helicates, which were only obtained by less constrained C
2
-orC
3
-linkers
(Figure 4.31) [70b].
Complete diastereoselectivity was also observed by M. Albrecht when studying a very
simple to prepare dicatechol imine ligand
72
(Figure 4.32), again introducing the chiral
information in the centre of the ligand [71]. Interestingly, this ligand has to undergo an
inversion of the cyclohexyl moiety to form the less favoured conformer with 1,2-diaxial
configured imine groups in order to be able to undergo diastereoselective self-assembly.
In a different approach he was able to derive dicatechol ligand
73
(Figure 4.33) with a
rather flexible ethylene linker with chiral 1-phenylethylamine moieties at the two ends.
Again this approach proved to be successful since only enantiomerically pure dinuclear
titanium helicates [Ti
2
(
73
)
3
] were obtained. Unfortunately, he was not able to assign an
absolute or relative stereochemistry of the metal centres in this case [72].
A completely different tetraanionic ligand was prepared by E.J. Corey in 1994. Starting
from mannitol he was able to prepare an enantiomerically pure tetrol ligand
74
that
formed a trinuclear titanium complex in a diastereoselective fashion, as revealed by
X-ray analysis (Figure 4.33) [73].









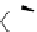







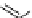
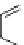












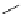



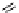

































































Search WWH ::

Custom Search