Chemistry Reference
In-Depth Information
Ph
2
PPP
h
2
Ph
Ph
65
Figure 4.28 Chiral P-donor ligand 65 with stereogenic P atoms.
4.7.7 Hydroxamic Acid Ligands
As shown by the naturally occurring complex formed upon coordination of rhodotorulic
acid to iron(III) ions, hydroxamic acids are suitable ligands for the formation of helicates.
In an effort to access artificial iron ion binders, A. Shanzer reported on a tertiary amine
66
carrying three identical chains containing a stereogenic centre and two hydroxamic
acid functions [68]. Upon coordination to iron(III) ions, this ligand was found to form an
optically pure dinuclear complex in a diastereoselective fashion (Figure 4.29).
4.7.8
b
-Diketonate Ligands
Another class of chelating ligands with a single negative charge are b-diketonates.
M. Albrecht prepared an example of these from tartaric acid (
67
) and demonstrated
that it can be used to achieve the diastereoselective formation of enantiomerically pure
neutral triple-stranded dinuclear iron(III) and gallium(III) helicates (Figure 4.30) [69].
Interestingly, this helicate was found to act as a non-covalently assembled cryptand for
lithium ions.
66
2 Fe
3+
Figure 4.29 Triamine 66 with chiral chains containing hydroxamic acid functionalities for
the complexation of iron(III) ions in a diastereoselective fashion (PM3-minimized structure, H
atoms omitted).



















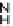



















Search WWH ::

Custom Search