Chemistry Reference
In-Depth Information
47
48
49
50
Figure 4.22 Enantiomerically pure bis(bipyridyl) ligands 47, 48, 49, and 50 explored by the
von Zelewski group to form cyclic (47), non-cyclic (48, 49), and polymeric (50) helicates via
diastereoselective self-assembly.
A. von Zelewsky then introduced “oligomers” and constitutional isomers of his famous
chiragen ligands (chiral 2,2
0
-bipyridines derived from terpenes like a-pinene) [48] to pre-
pare enantiomerically pure helicates. In a whole series of publications he could demonstrate
that using these ligands
47
-
50
(Figure 4.22) with the bicyclic terpene skeleton annealed to
one of the 2,2
0
-bipyridine rings is a very reliable and robust motif that ensures diastereose-
lective self-assembly of circular (
47
) [49], non-circular (
48
,
49
) [50], and even polymeric
helicates (
50
) [51]. Especially, the last one is exceptional because additional intermolecular
interaction in the solid state packing causes the formation of a polymeric aggregate to be
more favourable than the formation of discrete dinuclear coordination compounds.
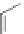
A very similar approach to that of A. von Zelewsky was propagated by the group of
E.C. Constable. They prepared chiral pinene-derived bis(bipyridine) ligands connected
by flexible alkyl bridges like
51
(Figure 4.23) and could show that these self-assembled
completely diastereoselectively to double-stranded helicates upon coordination to sil-
ver(I) ions despite the flexible linkers. However, when they investigated the coordina-
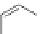
tion behaviour of these ligands towards copper(I) ions, they found a much more
51
53a
(R = Me)
53b
(R = Ph)
54
52
Figure 4.23 Enantiomerically pure bis(bipyridyl) ligands 51-54 explored by the groups of
E.C. Constable, N.C. Fletcher, and ourselves that were demonstrated to undergo diastereose-
lective self-assembly to optically pure helicates upon coordination to suitable transition metal
ions.



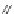





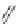









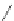
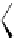
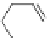


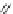


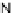


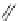
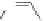



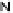





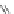

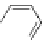

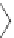
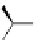


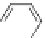

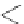






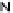
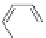










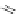


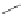


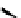





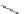




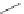



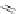









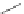







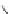
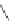



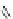





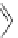

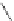


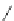






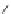


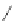
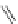

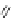










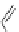




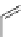







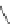










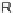





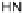




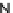

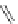


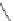




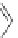



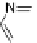

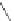
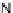
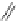
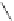















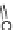

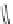





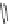







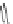



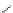

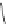

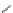










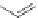














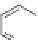








Search WWH ::

Custom Search