Chemistry Reference
In-Depth Information
(
R
)-
40
)-[Cu
2
{(
R
)-
40
)
2
]
2+
(
Δ
,
Δ
Figure 4.18 Diastereoselective self-assembly of an enantiomerically pure “meso”-helicate
(D,L)-[Cu
2
{(R)-40}
2
]
2þ
from 9,9
0
-spirobifluorene-bridged bis(bipyridine) ligand (R)-40.
and helicates are diastereomers and in fact there have been a number of studies to prepare
these
meso
-forms in a selective manner. In 1995 two articles were published within a
month about the selective formation of these
meso
-isomers. The first was from the group
of M. Albrecht who later found an interesting odd/even effect of the linker length between
two catechol units: odd numbers of methylene groups gave
meso
-helicates whereas even
numbers gave helicates (see also Figure 4.6) [7e,14a,b,38].
The second report came from the laboratory of M.M. Harding and she reported on a
number of naphthyl-bridged bis(bipyridine) ligands that rather gave rise to non-helical
metallomacrocycles rather than helicates upon coordination to nickel(II) or zinc(II) ions
[39]. Later, further studies were made by the groups of K.N. Raymond [40], K. Gloe [41],
and ourselves (Figure 4.18) [42] that further confirmed that
meso
-helicates can be
accessed in a selective manner.
A prediction, however, why the formation of
meso
-helicates becomes more favourable
than that of the corresponding helical diastereomers is still very difficult, except for the
systematically studied systems of M. Albrecht.
4.7 Diastereoselectivity II - Enantiomerically Pure Helicates from Chiral
Ligands
The previous example described an enantiomerically pure supramolecular aggregate. This
section will focus on the preparation and isolation of enantiomerically pure helicates. In
fact, there are two strategies to obtain helicates in optically pure form, either by resolution
of a racemic mixture or stereoselective self-assembly using enantiomerically pure
components.
The first is rather rare because the kinetic lability which is needed for the thermo-
dynamically controlled self-assembly to occur usually prevents the separation of enan-
tiomers. Thus, the resolution of racemic mixtures can only be successful with kinetically
quite stable assemblies that do not racemize under given conditions due to mechanisms
described in Section 4.2. One way to achieve this is by modifying the lability of a metal
complex by an electron transfer reaction, such as the oxidation of labile cobalt(II) ions to
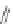
















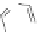









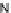




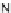









































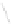











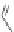
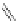












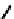








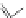


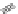
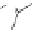
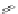

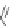




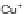




Search WWH ::

Custom Search