Chemistry Reference
In-Depth Information
[Cu{(
R
)-
27
}(Cl)H
2
O)]
[Cu{(
S
)-
27
}(Cl)H
2
O)]
[Cu
2
{(
R
)-
27
}
2
(ClO
4
)
2
]
[Cu
2
{(
S
)-
27
}
2
(ClO
4
)
2
]
Figure 4.14 Self-recognition process of racemic ligand 27 giving rise to a racemic mixture of
homochiral double-stranded helicates with non-stereogenic metal centres.
This behaviour is quite general if the ligand is properly designed and the forma-
tion of the helicate does not impose too much sterical stress on the assembly, which
finally overcomes the energetic differences between the possible stereoisomers. Rigid
bis(bipyridyl)ligands
28
-
33
based on 2,8-difunctionalized Tr
oger's base scaffolds, for
example, self-assemble in a completely diastereoselective self-recognition manner
into dinuclear double-stranded helicates upon coordination to copper(I) or silver(I)
ions (Figure 4.16) [33].
In a later study we were able to reveal the almost perfect preorganization of these lig-
ands for the formation of double-stranded helicates [34], but when we investigated the
formation of the corresponding dinuclear triple-stranded zinc(II) helicates or dinuclear
triple-stranded titanium(IV) complexes with corresponding di(catechol) ligands
34
and
35
we observed almost no selectivity because the rigid conformation of the ligand is just
not well preorganized to form these assemblies which ask for a greater opening angle of
the V-shaped ligand structure [35].
However, more flexible ligands
36
-
39
with a binaphthyl core unit that can adjust
better to adopt the conformations necessary for the formation of double-stranded
and
triple-stranded helicates were found to undergo a diastereoselective self-assembly of
both (Figure 4.17) [36].
€
(
R
,
R
)-
3
(
Δ
,
Δ
)-[Cu
2
{(
R
,
R
)-
3
}
2
]
2+
(
Δ
,
Δ
)-[Cu
2
{(
S
,
S
)-
3
}
2
]
2+
(
S
,
S
)-
3
Figure 4.15 Diastereoselective self-recognition process of racemic ligands (R,R)-3 and (S,S)-
3 giving rise to a racemic mixture of homochiral double-stranded helicates with stereogenic
metal centres.



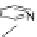
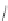
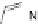









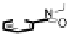


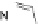





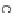








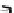





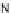




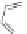




















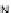









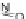









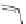



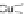


























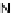





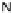
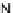

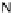











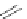
























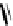



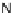


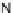
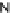
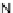
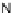








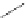

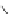
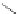


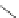


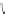

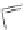







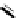











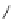

































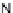
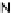













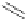




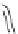





































Search WWH ::

Custom Search