Chemistry Reference
In-Depth Information
OO
O
O
O
O
Ti
Ti
OH
O
O
O
O
O
O
OH
O
O
O
OO
O
Ti
Ti
OO
O
O
OO
[Ti
2
(
9
)
3
]
n
4n-
OH
[Ti
2
(
9
)
3
]
4-
[Ti
2
(
9
)
2
(
10
)]
4-
2"Ti
4+
"
OH
9
-H
4
[Ti
2
(
10
)
3
]
n
4n-
base
OH
O
O
O
O
O
Ti
Ti
[Ti
2
(
9/10
)
3
]
n
4n-
OH
O
O
O
O
O
O
OH
O
O
O
O
O
O
Ti
OO
Ti
O
O
O
O
OH
[Ti
2
(
9
)(
10
)
2
]
4-
[Ti
2
(
10
)
3
]
4-
10
-H
4
[Ti
2
(
9
)
3
]
4-
[Ti
2
(
10
)
3
]
4-
+
Na
+
[Ti
2
(
9
)
3
]
n
4n-
[Ti
2
(
9
)
3
]
4-
1.5 equiv.
9
1.5 equiv.
10
2 equiv. "Ti
4+
"
K
+
+
+
[Ti
2
(
10
)
3
]
4-
[Ti
2
(
10
)
3
]
4-
Li
+
[Ti
2
(
9
)
3
]
4-
+ [Ti
2
(
10
)
3
]
4-
+ [Ti
2
(
9
)
2
(
10
)]
4-
Figure 4.6 Counterion template effects II. Alkali metal ions act as templates in the formation
of helicates from bis(catecholate) ligands. Top: possible homoleptic {[Ti
2
(9)
3
]
4
and
[Ti
2
(10)
3
]
4
} and heteroleptic {[Ti
2
(9)
2
(10)]
4
and [Ti
2
(9)(10)
2
]
4
} dinuclear and oligomeric
complexes obtained from a 1 : 1 mixture of ligands 9-H
4
and 10-H
4
and titanium(IV) ions in
the presence of alkali metal carbonates (2 eq.) as base. Bottom: detected species obtained
with different cations and mixtures of cations.
with at least two different metal binding sites. In doing so, one can make use of the prefer-
ence of different metal ions for different donor atoms, different coordination numbers, or
charges in order to achieve selectivity.
The first reports on this came from the laboratory of C. Piguet in 1995 [16] who could
nicely demonstrate that heteronuclear complexes containing d- and f-block metal ions can
be assembled in a selective fashion by using benzimidazole ligands like
12
and
13
depicted in Figure 4.8. Since then the group of C. Piguet has characterized quite a number




















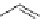






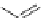

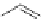








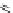
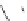




































































































































Search WWH ::

Custom Search