Chemistry Reference
In-Depth Information
9
⊕
N
N
N
N
N
F
e
N
N
N
5 FeCl2,
ethylene glycol
170ºC
N
N
N
N
N
N
Fe
Fe
N
N
N
5
N
N
N
N
N
Cl
N
N
N
N
N
N
N
F
e
Fe
N
N
N
N
N
N
N
[Fe
5
(
8
)
5
Cl]
9+
8
Figure 4.5 Counterion template effects I. Chloride ions act as templates in the formation of
circular helicates.
In the same year M. Albrecht found the first indications that alkali metal ions can also
have a pronounced templating effect in the formation of dinuclear complexes of bis(cat-
echolate) ligands, as only the use of lithium ions resulted in the formation of well defined
dinuclear complexes from ligand
9
(in this case
meso
-helicates which will be discussed in
more detail below), as shown by NMR spectroscopy and X-ray crystallography, whereas
sodium and potassium ions gave complicated mixtures of aggregates with this ligand
alone [14a]. In a series of following studies he was able to find more evidence that this
effect was quite general for this kind of (formal) anionic helicates [14]. Interestingly, a
template effect was also observed when a mixture of ligands
9
and
10
was investigated
which usually gave rise to either
meso
-helicates [M
2
(
9
)
3
]
n
or helicates [M
2
(
10
)
3
]
n
,
respectively (Figure 4.6) [14c].
Later, K.N. Raymond found that this counterion effect can also be extended to a com-
plete structural interconversion between triple-stranded helicates and capsular tetrahedral
cluster aggregates by host-guest interactions with alkylammonium ions (Figure 4.7) [15].
4.4 Sequence Selectivity
So far, almost all ligands that were used to make helicates were symmetrical. Only one of
K.N. Raymonds ligands used in his structural dynamics studies [10] was non-symmetrical
because of two different terminal substituents at the catechol units. This difference, how-
ever, did not result in a sequence selective formation of a single helicate but rather
resulted in a complex mixture of all possible isomers. In order to achieve sequence selec-
tivity it was found to be a better approach to use heterotopic ligand strands, that is ligands
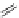


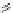



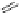


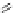


























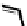













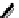


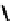







































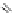






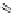





















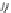
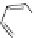



















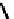
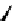



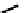













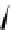

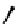
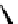







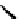



















































































Search WWH ::

Custom Search