Chemistry Reference
In-Depth Information
O
O
(a)
N
H
N
H
Ga
Ga
2 [Ga(H
2
O)
6
]
3+
+
3
O
-
-
O
+
12 H
2
O
O
-
O
L
4
-
[Ga
2
L
3
]
6-
Point group
O
h
24
2
6
1
C
2v
2
1
1
D
3
6
1
1/2
C
2v
2
1
1
σ
ext
σ
int
σ
mix
3
σ
tot
1536
2
2
(1536)
2
· 2
3
Ga,
L
=
= 1536
ω
2,3
3 · 2
12
C
C
B1
B2
(b)
B1
B2
B1
B2
C
C
A
4
B2
B1
A
4
A
5
A
5
B1
B2
A
3
A
3
B1
B2
A
1
A
2
A
1
C
C
A
6
A
6
A
2
B2
B1
B2
B1
B1
B2
C
C
B1
B2
8 possibilities
3 possibilities
8 possibilities
B1
B2
B2
B1
4 possibilities
2 possibilities (two enantiomers)
l
r
2·8·3·8·4
Ga,
L
ω
2,3
=
= 1536
1
Figure 3.6 Application of (a) the symmetry number method and (b) the direct counting
method for calculating statistical factors in [Ga
2
L
3
]
6
.
[13] Reproduced by permission of The
Royal Society of Chemistry.
intermolecular,
intramolecular.
All these situations may occur in a helicate assembly and will be reviewed in
detail below.
intermolecular
!
intramolecular,
intramolecular
!
3.3.1 Allosteric Cooperativity
The well understood concept of allosteric cooperativity is often applied in biology for
studying protein-ligand interactions strictly relying on intermolecular processes [27,28].
This type of cooperativity corresponds to the behaviour, where the initial binding of a

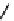
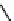

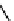

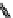






















































































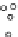
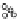

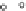


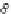









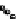




































































Search WWH ::

Custom Search