Biomedical Engineering Reference
In-Depth Information
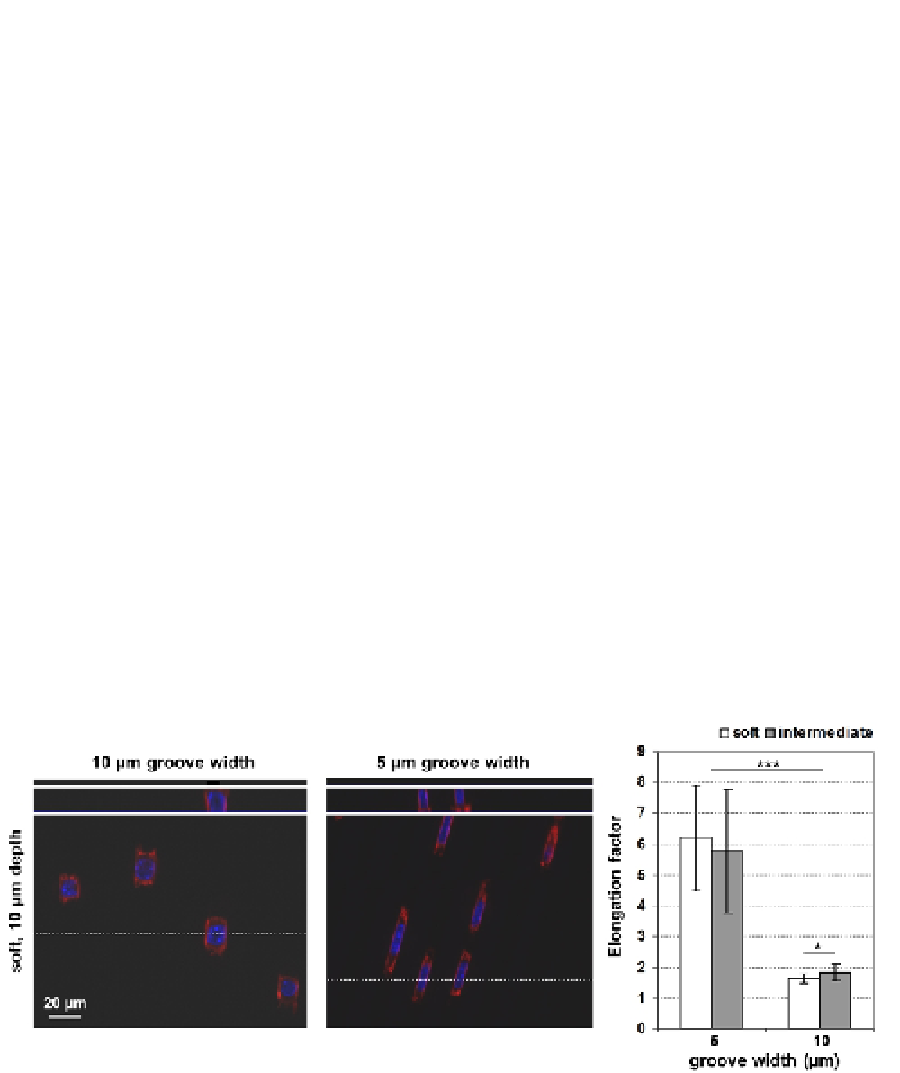
The cells were found to adhere inside the grooves and form adhesion contacts with the side
walls as well as to the bottom of the shallower grooves. They were able to adhere to the
narrower (5 µm wide) grooves as well, but had to undergo tremendous shape adaptations,
including deformation of the cell nucleus, as observed from fluorescence microscopy using
selective staining agents for the actin cytoskeleton and for the nucleus (
Figure 9
, right
image). The cells apparently like to snug into well-fitting grooves in a more compliant
hydrogel material. No visual deformation of the hydrogels was found; the cells rather
adapted their shape to fit into too narrow grooves (i.e. 5 µm wide; Schulte et al., 2010).
Second, we investigated whether proteins could adsorb non-specifically to the gels and if so,
if they would adsorb differently on the topographic structures, e.g. preferentially on the
walls of the grooves, or on the convex (outer) or concave (inner) corners, since the
eventually adherent cells were found to be located inside of the grooves and aligned along
the ridges. We incubated the patterned hydrogel samples in protein solutions of three
selected extracellular matrix (ECM) proteins, i.e. Fibronectin (FN), Vitronectin (VN) and
bovine serum albumin (BSA). The former two are cell adhesion mediating proteins, while
BSA does not facilitate cell adhesion. BSA is the most abundant protein in serum, and
besides its abundance it is also a small protein, which diffuses fast and reaches the surface
faster than the larger proteins FN and VN and finally the cells.
With help of immunological staining using fluorescently labeled antibodies we could
demonstrate that from pure protein solutions all three proteins were able to adsorb to the
PEG surfaces to a certain extent (Schulte et al., 2011). The fluorescence was homogeneously
distributed over the surface; there was no detectable difference between for example the
vertical walls or the horizontal planes between the grooves. Finally, it seemed that BSA was
able to diffuse into the PEG hydrogels, since the fluorescence was not restricted to the
surface (Schulte et al., 2011).
Fig. 9. Elongated cell morphology on topographically patterned PEG hydrogels with 10 or 5
µm wide grooves. Reprinted with permission from: Schulte et al.
Biomacromolecules
,
11,
3375-
83. Copyright 2010 American Chemical Society.
Notwithstanding the ability of all three proteins to adsorb to the PEG hydrogel, only VN
was observed to adsorb in a detectable amount under competitive conditions, i.e. from a
mixture of VN and FN and when serum, a complex mixture of proteins, was supplemented
(
Table 1
; Schulte et al., 2011). This result was rather unexpected, since BSA is usually