Biomedical Engineering Reference
In-Depth Information
4.2 Tissue crosslinking after carboxylic group activation
Due to the problems associated with the use of glutaraldehyde, various non-aldehyde
alternative methods have been developed to stabilize and post-treat tissues. The
crosslinking agents used in collagen-rich biomaterials can use both primary amino groups
and acid groups of polypeptide chains. Historically, a water soluble carbodiimide (1-ethyl-3-
(3-dimethyl amino propyl) carbodiimide / EDAC) was first used for the modification of
carboxylic groups in proteins for peptide synthesis (Sheehan & Hlavka, 1956) and to
promote crosslinking in gelatin (Sheehan & Hlavka, 1957).
The mechanism for the reaction between carboxylic groups and EDAC leading to amide
bond formation is as follows: The addition of a carboxylic acid diimide produces an isourea
ester, an O-acyl isourea. The intermediate O-acyl isourea is an activated carboxylic acid
derivative with similar reactivity to an anhydride or acyl halide, and can be subjected to a
subsequent nucleophilic substitution by an amine yielding a dialkyl amide and urea
(Carraway & Khosland, 1972). Because carbodiimide is just a coupling agent, when used to
crosslink collagen in the absence of agents with dual functionality, only promotes the
formation of an amide bond between carboxylic acid and amino reactive groups present in
the tissue, as depicted in figure 6.
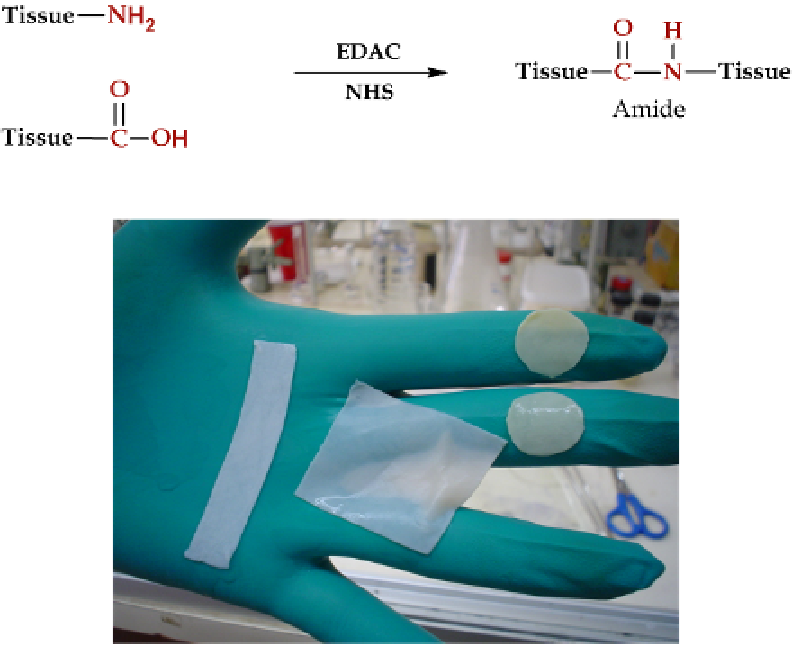
Fig. 6. Schematic representation of tissue crosslinking with EDAC and NHS
Fig. 7. Bovine perichardial tissue crosslinked with EDAC (rectangules) or glutaraldehyde
(circles)
This requires that the activated carboxylic groups be close enough to the amino groups to
achieve direct bonding (amide bond formation). The carbodiimides are hydrolyzed rapidly