Biomedical Engineering Reference
In-Depth Information
O
−
M …
O
−
M …
O
−
M …
O
−
M …
H
2
N
−
(CH
2
)
3
−
Si
−
H
2
N
−
(CH
2
)
3
−
Si
−
O
−
M …
a
= 0
O
−
M …
a
= 0
H
2
N
−
(CH
2
)
3
−
Si
−
H
2
N
−
(CH
2
)
3
−
Si
−
O
−
M …
a
= 0.5
O
−
M …
a
= 0.5
O
−
M …
O
−
M …
O
−
Si …
O
−
Si …
O
−
M …
O
−
M …
O
−
Si …
O
−
Si …
H
2
N
−
(CH
2
)
3
−
Si
−
H
2
N
−
(CH
2
)
3
−
Si
−
O
−
Si …
a
= 1
O
−
Si …
a
= 1
H
2
N
−
(CH
2
)
3
−
Si
−
H
2
N
−
(CH
2
)
3
−
Si
−
O
−
Si …
a
= 1.5
O
−
Si …
a
= 1.5
O
−
Si …
O
−
Si …
O
−
Si …
O
−
Si …
O
−
M …
O
−
M …
O
−
Si …
O
−
Si …
H
2
N
−
(CH
2
)
3
−
Si
−
H
2
N
−
(CH
2
)
3
−
Si
−
O
−
Si …
a
= 1.5
O
−
Si …
a
= 1.5
H
2
N
−
(CH
2
)
3
−
Si
−
H
2
N
−
(CH
2
)
3
−
Si
−
O
−
Si …
a
= 2
O
−
Si …
a
= 2
OH
OH
OH
OH
Fig. 6. Possible products of APTES reaction. “M” designates metal elements of stainless steel.
0.4
0.4
a
= 0
a
= 1
0.3
0.3
0.2
0.2
0.1
0.1
R
2
= 0.02
R
2
= 0.53
0.0
0.0
0.0
0.1
0.2
0.3
0.4
0.0
0.1
0.2
0.3
0.4
0.4
0.4
a
= 1.5
a
= 2
0.3
0.3
0.2
0.2
0.1
0.1
R
2
= 0.68
R
2
= 0.80
0.0
0.0
0.0
0.1
0.2
0.3
0.4
0.0
0.1
0.2
0.3
0.4
(Fe
ox
+ Cr
ox
) /
Σ
org
(Fe
ox
+ Cr
ox
) /
Σ
org
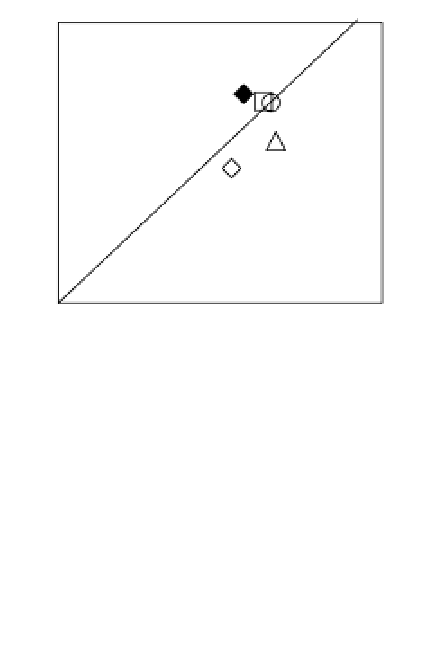
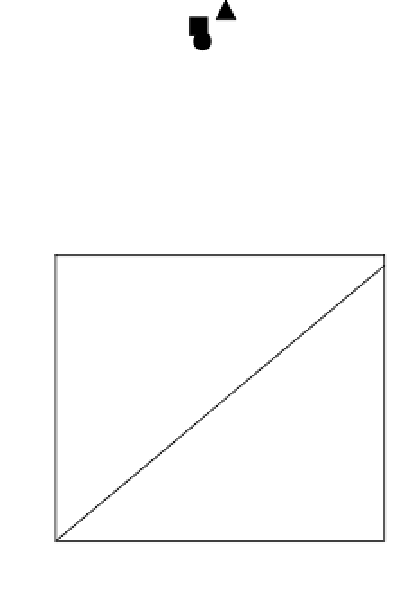
Fig. 7. Relations between molar concentrations ratioed to the sum of organic elements (Σorg)
measured by XPS at θ = 0° (data from Table 2) on native (open symbols) or silanized
stainless steel (closed symbols), as such (
¡
,
) or further treated with coupling agent BS
(
S
,
U
), glucose oxidase (
z
,
{
) or coupling agent followed by glucose oxidase (
,
).