Biology Reference
In-Depth Information
Major phases of biomarker development
The development of biomarkers entails a number of phases, from the identi-
fication of promising molecular targets to longitudinal clinical trials in asso-
ciation with a specific treatment. Three major steps are required to develop a
clinical test for screening. First, the discovery or pilot phase compares 20 to 40
cases and controls using antibody-based arrays or MS-based approaches as
discussed previously. It is recommended that the term “candidate biomarker”
or “potential biomarker” be used to refer to findings of early phase studies
when additional validation is needed. Next, the validation phase is usually
performed with immunoassays rather than MS, and the sample set is created
from a retrospective longitudinal case-control repository. This process should
be done on a training set followed by an independent validation set; valida-
tion using sets from multiple institutions is ideal. The final step focuses on a
few biomarkers and requires a prospective multicenter validation, typically on
thousands of samples before the release of the clinical test. For high-through-
put purposes and standardization between laboratories, only immunoas-
says are used at this step. If the blood test detects disease early before clinical
signs become apparent, both a screen-positive rule and a false-screen rate are
defined. It is hoped that this step will lead to a clinical test that will be approved
by the U.S. Food and Drug Administration. Then, the impact of the screen on
the reduction of disease burden on the population of interest is quantified
[33]
.
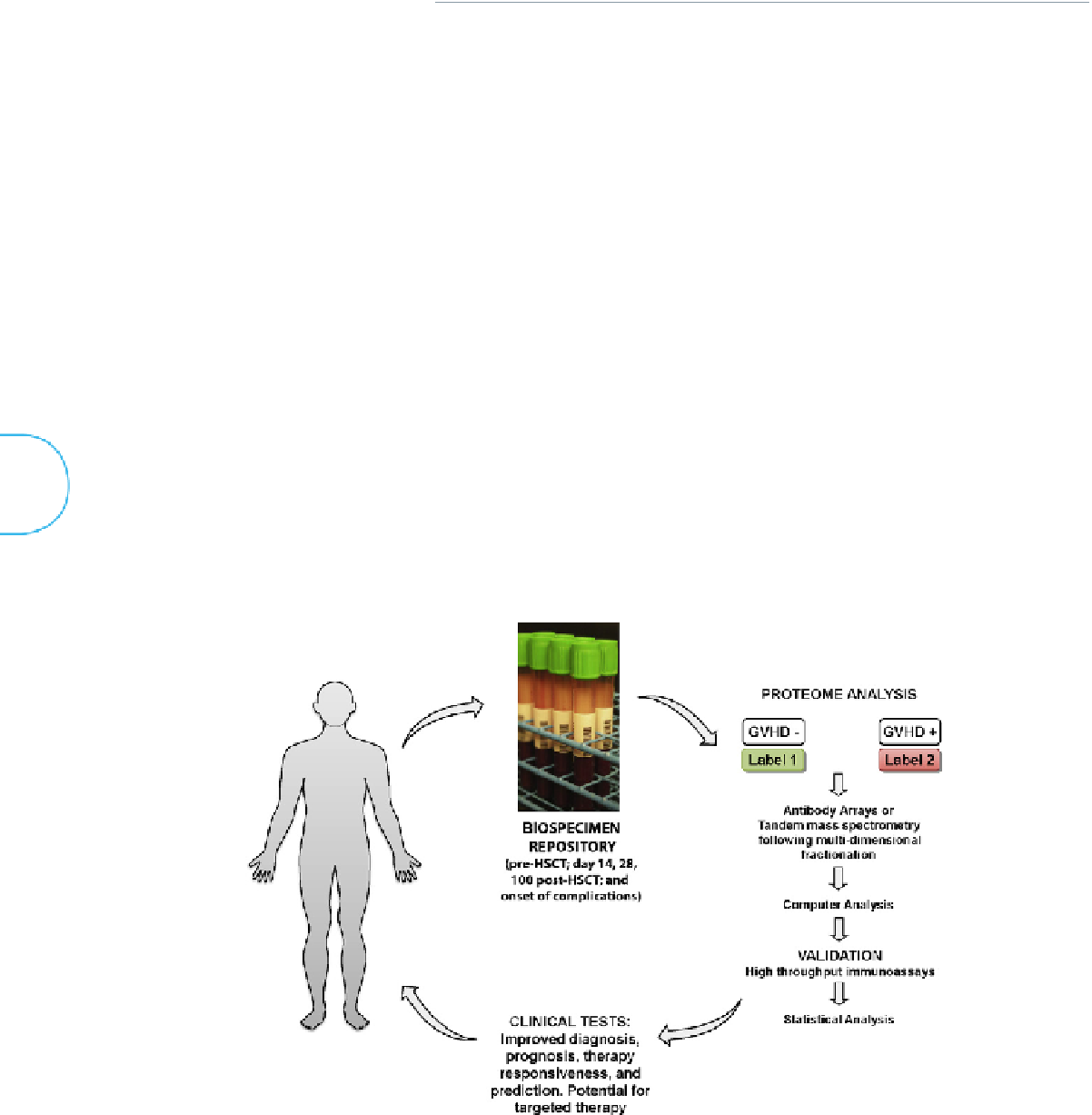
Figure 19.2
shows the workflow of biomarker development post-HSCT.
456
FIGURE 19.2
Clinical proteomics: from the bedside to the bench and back to the bedside for personalized therapy of GVHD post-HSCT. Biospecimens (body fluids such as
plasma, serum, or urine) are collected on a calendar- and event-driven schedule. Their proteomes are analyzed in detail with technologies that permit in-depth identifica-
tion and quantification of candidate proteins that are significantly altered in GVHD. This step is called the discovery phase. Upon validation with high-throughput im-
munoassays in a large series of patients and independent sets of patients, these can be considered biomarkers that could move into clinics. The clinical tests could help
clinicians to better monitor their patients thanks to blood tests that improve diagnosis, prognosis, responsiveness to therapy, and prediction of occurrence of the disease.
In addition to adapting the current therapy with risk stratification, some of these biomarkers are drug-targetable and could help develop GVHD-specific drugs.
Search WWH ::

Custom Search